| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 03:14:10 UTC |
|---|
| Update Date | 2016-11-09 01:17:18 UTC |
|---|
| Accession Number | CHEM021204 |
|---|
| Identification |
|---|
| Common Name | Bromohexine |
|---|
| Class | Small Molecule |
|---|
| Description | A substituted aniline that is 2,4-dibromoaniline which is substituted at position 6 by a methyl group. It is used (as the monohydrochloride salt) as a mucolytic for the treatment of respiratory disorders associated with productive cough (i.e. a cough characterised by the production of sputum). |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- Suspected Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
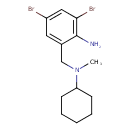
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,5-Dibromo-N(alpha)-cyclohexyl-N(alpha)-methyltoluene-alpha-2-diamine | ChEBI | | Bromhexina | ChEBI | | Bromhexinum | ChEBI | | Fluibron | ChEBI | | N-Cyclohexyl-N-methyl-(2-amino-3,5-dibrombenzyl)amine | ChEBI | | 3,5-Dibromo-N(a)-cyclohexyl-N(a)-methyltoluene-a-2-diamine | Generator | | 3,5-Dibromo-N(α)-cyclohexyl-N(α)-methyltoluene-α-2-diamine | Generator | | Berlin-chemie brand OF bromhexine hydrochloride | MeSH | | Boehrvet brand OF bromhexine hydrochloride | MeSH | | Bromhexin berlin-chemie | MeSH | | Bromhexin-ratiopharm | MeSH | | Bromhexine, famel | MeSH | | Flubron | MeSH | | Hoechst brand OF bromhexine hydrochloride | MeSH | | Krewel brand OF bromhexine hydrochloride | MeSH | | Merckle brand OF bromhexine hydrochloride | MeSH | | Monohydrochloride, bromhexine | MeSH | | Mucohexine | MeSH | | Novartis brand OF bromhexine hydrochloride | MeSH | | SB CH Brand OF bromhexine hydrochloride | MeSH | | SB-CH Brand OF bromhexine hydrochloride | MeSH | | Bromhexin von CT | MeSH | | CT Arzneimittel brand OF bromhexine hydrochloride | MeSH | | Aparsonin | MeSH | | BC, Bromhexin | MeSH | | Bisolvon | MeSH | | Bromhexin BC | MeSH | | DurElix | MeSH | | Hustentabs ratiopharm | MeSH | | Hustentabs-ratiopharm | MeSH | | Hustentabsratiopharm | MeSH | | Quentan | MeSH | | CT, Bromhexin von | MeSH | | CT-Arzneimittel brand OF bromhexine hydrochloride | MeSH | | Von CT, bromhexin | MeSH | | Apex brand OF bromhexine hydrochloride | MeSH | | Berlin chemie brand OF bromhexine hydrochloride | MeSH | | Boots brand OF bromhexine hydrochloride | MeSH | | Bromhexin berlin chemie | MeSH | | Bromhexin ratiopharm | MeSH | | Bromhexine monohydrochloride | MeSH | | Dur-elix | MeSH | | Fher brand OF bromhexine hydrochloride | MeSH | | Hydrochloride, bromhexine | MeSH | | Omniapharm brand OF bromhexine hydrochloride | MeSH | | Tesacof | MeSH | | 3m Brand OF bromhexine hydrochloride | MeSH | | Boehringer ingelheim brand OF bromhexine hydrochloride | MeSH | | Bromhexin | MeSH | | Bromhexin berlinchemie | MeSH | | Bromhexine hydrochloride | MeSH | | Bromhexinratiopharm | MeSH | | Brotussol | MeSH | | Darolan | MeSH | | Dur elix | MeSH | | Famel bromhexine | MeSH | | Flegamin | MeSH | | promeco Brand OF bromhexine hydrochloride | MeSH | | Ratiopharm brand OF bromhexine hydrochloride | MeSH |
|
|---|
| Chemical Formula | C14H20Br2N2 |
|---|
| Average Molecular Mass | 376.130 g/mol |
|---|
| Monoisotopic Mass | 373.999 g/mol |
|---|
| CAS Registry Number | 3572-43-8 |
|---|
| IUPAC Name | 2,4-dibromo-6-{[cyclohexyl(methyl)amino]methyl}aniline |
|---|
| Traditional Name | bromhexine |
|---|
| SMILES | CN(CC1=CC(Br)=CC(Br)=C1N)C1CCCCC1 |
|---|
| InChI Identifier | InChI=1S/C14H20Br2N2/c1-18(12-5-3-2-4-6-12)9-10-7-11(15)8-13(16)14(10)17/h7-8,12H,2-6,9,17H2,1H3 |
|---|
| InChI Key | OJGDCBLYJGHCIH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylmethylamines. Phenylmethylamines are compounds containing a phenylmethtylamine moiety, which consists of a phenyl group substituted by an methanamine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylmethylamines |
|---|
| Direct Parent | Phenylmethylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzylamine
- Phenylmethylamine
- 2-bromoaniline
- Aniline or substituted anilines
- Bromobenzene
- Cyclohexylamine
- Halobenzene
- Aralkylamine
- Aryl bromide
- Aryl halide
- Tertiary amine
- Tertiary aliphatic amine
- Primary amine
- Organopnictogen compound
- Organonitrogen compound
- Organobromide
- Organohalogen compound
- Amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-03di-0095000000-2c4f15e31beb22fd1178 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-03di-0090000000-4d3be1605bfa79609959 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-03di-0090000000-428c05c13a2a9e326945 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-03di-0390000000-b0ece5d5d6f8b78af198 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1039000000-996bb07339c84bf0b0a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-3093000000-ddfc9253090e76c0b559 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01q9-3090000000-307fd61d87cf01d30457 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0109000000-dad89f7da3b48cfd0d02 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-1349000000-8b7120c4327c08f80596 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-7690000000-e3e7d1043644884bc5e8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB09019 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Bromhexine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 77032 |
|---|
| PubChem Compound ID | 2442 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|