| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 07:19:04 UTC |
|---|
| Update Date | 2016-11-09 01:16:15 UTC |
|---|
| Accession Number | CHEM020679 |
|---|
| Identification |
|---|
| Common Name | Dabigatran etexilate |
|---|
| Class | Small Molecule |
|---|
| Description | An aromatic amide obtained by formal condensation of the carboxy group of 2-{carbamimidoyl}phenyl)amino]methyl}-1-methyl-1H-benzimidazole-5-carboxylic acid with the secondary amino group of ethyl N-pyridin-2-yl-beta-alaninate. A prodrug for dabigatran, a thrombin inhibitor and anticoagulant which is used for the prevention of stroke and systemic embolism. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
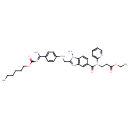
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Pradaxa | Kegg | | Dabigatran etexilic acid | Generator | | Dabigatran | HMDB | | Ethyl 3-[[[4-[[[(hexyloxyl)carbonyl]amino]iminomethyl]phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl](pyridin-2-yl)amino] propanoate | HMDB | | Etexilate mesylate, dabigatran | HMDB | | Etexilate, dabigatran | HMDB | | BIBR 1048 | HMDB | | Mesylate, dabigatran etexilate | HMDB | | N-((2-(((4-(Aminoiminomethyl)phenyl)amino)methyl)-1-methyl-1H-benzimidazol-5-yl)carbonyl)-N-2-pyridinyl-beta-alanine | HMDB | | Dabigatran etexilate mesylate | HMDB |
|
|---|
| Chemical Formula | C34H41N7O5 |
|---|
| Average Molecular Mass | 627.733 g/mol |
|---|
| Monoisotopic Mass | 627.317 g/mol |
|---|
| CAS Registry Number | 211915-06-9 |
|---|
| IUPAC Name | ethyl 3-(1-{2-[({4-[amino({[(hexyloxy)carbonyl]imino})methyl]phenyl}amino)methyl]-1-methyl-1H-1,3-benzodiazol-5-yl}-N-(pyridin-2-yl)formamido)propanoate |
|---|
| Traditional Name | dabigatran etexilate |
|---|
| SMILES | CCCCCCOC(=O)N=C(N)C1=CC=C(NCC2=NC3=C(C=CC(=C3)C(=O)N(CCC(=O)OCC)C3=CC=CC=N3)N2C)C=C1 |
|---|
| InChI Identifier | InChI=1S/C34H41N7O5/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44) |
|---|
| InChI Key | KSGXQBZTULBEEQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzimidazoles. These are organic compounds containing a benzene ring fused to an imidazole ring (five member ring containing a nitrogen atom, 4 carbon atoms, and two double bonds). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzimidazoles |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzimidazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzimidazole
- Aniline or substituted anilines
- Phenylalkylamine
- Secondary aliphatic/aromatic amine
- Aralkylamine
- Monocyclic benzene moiety
- N-substituted imidazole
- Pyridine
- Benzenoid
- Imidolactam
- Azole
- Imidazole
- Tertiary carboxylic acid amide
- Heteroaromatic compound
- Carboxylic acid ester
- Carboxamide group
- Carbonic acid derivative
- Amino acid or derivatives
- Monocarboxylic acid or derivatives
- Amidine
- Carboxylic acid amidine
- Organic 1,3-dipolar compound
- Carboxylic acid derivative
- Azacycle
- Secondary amine
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Organic oxygen compound
- Amine
- Organonitrogen compound
- Carbonyl group
- Organic nitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001v-3234390000-c2177c303cbef52afbdf | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-004r-0292116000-55b368cbe92da888e681 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00kk-0981100000-78856b63936cf67ebce2 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-004r-0292116000-55b368cbe92da888e681 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ufs-4720194000-b25f304a4eefb48ffb14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uds-9634660000-7ac6e0cd9b01e5cd8430 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fba-4942200000-823214c2258288f41cbb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00e9-2200592000-91bb2cde7f5a1c074cff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-008d-3500940000-5f6725f159c9fa1d9ac1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-4504930000-e756884bbf82c6cfced2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000019000-b01a3a5bf142faf27699 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004l-0001960000-f016ad334f1f10cd9561 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-005i-3913404000-8193e59d4a4ff6967cd9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000259000-c2af6ee3f89a87dfae64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-1000921000-06293e9a87d653604565 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fk9-1001900000-49277b2224b211f8de10 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06695 |
|---|
| HMDB ID | HMDB0015641 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Dabigatran |
|---|
| Chemspider ID | 4948999 |
|---|
| ChEBI ID | 70746 |
|---|
| PubChem Compound ID | 213023 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=21265583 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=21429525 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=21542663 | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=21748501 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=21859976 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=21881389 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=22252796 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=22276081 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=22298812 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=22318514 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=22348256 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=22422743 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=22431533 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=22488474 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=22536678 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=22552763 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=22563715 | | 18. https://www.ncbi.nlm.nih.gov/pubmed/?term=22564134 | | 19. https://www.ncbi.nlm.nih.gov/pubmed/?term=22669799 | | 20. https://www.ncbi.nlm.nih.gov/pubmed/?term=22782539 | | 21. https://www.ncbi.nlm.nih.gov/pubmed/?term=22803489 | | 22. https://www.ncbi.nlm.nih.gov/pubmed/?term=22995531 | | 23. https://www.ncbi.nlm.nih.gov/pubmed/?term=23476049 | | 24. Eriksson BI, Dahl OE, Buller HR, Hettiarachchi R, Rosencher N, Bravo ML, Ahnfelt L, Piovella F, Stangier J, Kalebo P, Reilly P: A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005 Jan;3(1):103-11. | | 25. Stangier J, Eriksson BI, Dahl OE, Ahnfelt L, Nehmiz G, Stahle H, Rathgen K, Svard R: Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005 May;45(5):555-63. | | 26. Di Nisio M, Middeldorp S, Buller HR: Direct thrombin inhibitors. N Engl J Med. 2005 Sep 8;353(10):1028-40. | | 27. Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Kalebo P, Christiansen AV, Hantel S, Hettiarachchi R, Schnee J, Buller HR: Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007 Nov;5(11):2178-85. | | 28. Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Buller HR: Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007 Sep 15;370(9591):949-56. | | 29. Ezekowitz MD, Reilly PA, Nehmiz G, Simmers TA, Nagarakanti R, Parcham-Azad K, Pedersen KE, Lionetti DA, Stangier J, Wallentin L: Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol. 2007 Nov 1;100(9):1419-26. Epub 2007 Aug 17. | | 30. Ginsberg JS, Davidson BL, Comp PC, Francis CW, Friedman RJ, Huo MH, Lieberman JR, Muntz JE, Raskob GE, Clements ML, Hantel S, Schnee JM, Caprini JA: Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009 Jan;24(1):1-9. doi: 10.1016/j.arth.2008.01.132. Epub 2008 Apr 14. | | 31. Wolowacz SE, Roskell NS, Plumb JM, Caprini JA, Eriksson BI: Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost. 2009 Jan;101(1):77-85. | | 32. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L: Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139-51. doi: 10.1056/NEJMoa0905561. Epub 2009 Aug 30. | | 33. Bauer KA: New oral anticoagulants in development: potential for improved safety profiles. Rev Neurol Dis. 2010;7(1):1-8. | | 34. Scaglione F: New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013 Feb;52(2):69-82. doi: 10.1007/s40262-012-0030-9. | | 35. European Medicines Agency: http://www.ema.europa.eu/humandocs/PDFs/EPAR/pradaxa/H-829-en6.pdf | | 36. Abrams P and Marzella N: Dabigatran (Rendix): A Promising New Oral Direct Thrombin Inhibitor. Drug Forecast. 2007;32(5):271-5.: http://www.pharmscope.com/ptjournal/fulltext/32/5/PTJ3205271.pdf |
|
|---|