| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 07:18:41 UTC |
|---|
| Update Date | 2016-11-09 01:16:14 UTC |
|---|
| Accession Number | CHEM020669 |
|---|
| Identification |
|---|
| Common Name | Prucalopride |
|---|
| Class | Small Molecule |
|---|
| Description | Prucalopride is a dihydrobenzofurancarboxamide derivative from the benzofurane family that selectively stimulates 5-HT4 receptors and thus, it presents enterokinetic properties.[A37348] The high selectivity of prucalopride allowed further development as it prevented the cardiac adverse reactions observed due to non-target effects of precedent therapies.[A40254] Prucalopride was developed by Shire Development LLC and approved for use in Europe in 2009,[A40250] in Canada on December 7, 2011 and by the FDA on December 17, 2018.[L4880] |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
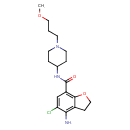
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| R-093877Prucalopride | ChEMBL | | Resotrans | MeSH | | Resolor | MeSH | | Resotran | MeSH | | 4-Amino-5-chloro-N-[1-(3-methoxypropyl)piperidin-4-yl]-2,3-dihydro-1-benzofuran-7-carboximidate | Generator | | 4-Amino-5-chloro-N-(1-(3-methoxypropyl)-4-piperidinyl)-2,3-dihydro-1-benzofuran-7-carboxamide | MeSH |
|
|---|
| Chemical Formula | C18H26ClN3O3 |
|---|
| Average Molecular Mass | 367.870 g/mol |
|---|
| Monoisotopic Mass | 367.166 g/mol |
|---|
| CAS Registry Number | 179474-81-8 |
|---|
| IUPAC Name | 4-amino-5-chloro-N-[1-(3-methoxypropyl)piperidin-4-yl]-2,3-dihydro-1-benzofuran-7-carboxamide |
|---|
| Traditional Name | prucalopride |
|---|
| SMILES | COCCCN1CCC(CC1)NC(=O)C1=C2OCCC2=C(N)C(Cl)=C1 |
|---|
| InChI Identifier | InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23) |
|---|
| InChI Key | ZPMNHBXQOOVQJL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aminobenzamides. These are organic compounds containing a benzamide moiety with an amine group attached to the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Aminobenzamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzamide
- Coumaran
- Alkyl aryl ether
- Aryl chloride
- Aryl halide
- Piperidine
- Amino acid or derivatives
- Carboxamide group
- Secondary carboxylic acid amide
- Tertiary aliphatic amine
- Tertiary amine
- Oxacycle
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Azacycle
- Organoheterocyclic compound
- Organohalogen compound
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Organochloride
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9812000000-f1bd170ac71ec919b417 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0309000000-de7dc5c7c5e8b2bd381c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fs-4926000000-bc02ec523ae005321132 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ym-8900000000-261664a7dcbdfbafb0b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0109000000-463adb3809c2c0b711fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-3849000000-c813744bf4a58d6f13da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02td-4960000000-57970845cde3ad05051c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06480 |
|---|
| HMDB ID | HMDB0256880 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Prucalopride |
|---|
| Chemspider ID | 2314539 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|