| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 07:18:23 UTC |
|---|
| Update Date | 2016-11-09 01:16:14 UTC |
|---|
| Accession Number | CHEM020660 |
|---|
| Identification |
|---|
| Common Name | Clevidipine |
|---|
| Class | Small Molecule |
|---|
| Description | Clevidipine is a dihydropyridine L-type calcium channel blocker that is selective for vascular smooth muscle and is indicated for blood pressure reduction when oral therapy is not an option. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
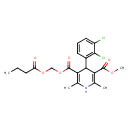
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Clevidipine butyrate | Kegg | | Cleviprex | Kegg | | Clevidipine butyric acid | Generator | | Butyroxymethyl methyl 4-(2',3'-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate | MeSH | | Methyl 5-{[(butanoyloxy)methoxy]carbonyl}-4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxylic acid | Generator |
|
|---|
| Chemical Formula | C21H23Cl2NO6 |
|---|
| Average Molecular Mass | 456.316 g/mol |
|---|
| Monoisotopic Mass | 455.090 g/mol |
|---|
| CAS Registry Number | 167221-71-8 |
|---|
| IUPAC Name | methyl 5-{[(butanoyloxy)methoxy]carbonyl}-4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxylate |
|---|
| Traditional Name | methyl 5-{[(butanoyloxy)methoxy]carbonyl}-4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxylate |
|---|
| SMILES | CCCC(=O)OCOC(=O)C1=C(C)NC(C)=C(C1C1=CC=CC(Cl)=C1Cl)C(=O)OC |
|---|
| InChI Identifier | InChI=1S/C21H23Cl2NO6/c1-5-7-15(25)29-10-30-21(27)17-12(3)24-11(2)16(20(26)28-4)18(17)13-8-6-9-14(22)19(13)23/h6,8-9,18,24H,5,7,10H2,1-4H3 |
|---|
| InChI Key | KPBZROQVTHLCDU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dihydropyridinecarboxylic acids and derivatives. Dihydropyridinecarboxylic acids and derivatives are compounds containing a dihydropyridine moiety bearing a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Hydropyridines |
|---|
| Direct Parent | Dihydropyridinecarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydropyridinecarboxylic acid derivative
- Tricarboxylic acid or derivatives
- 1,2-dichlorobenzene
- Chlorobenzene
- Acylal
- Fatty acid ester
- Halobenzene
- Aryl chloride
- Aryl halide
- Monocyclic benzene moiety
- Fatty acyl
- Benzenoid
- Vinylogous amide
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Methyl ester
- Amino acid or derivatives
- Carboxylic acid ester
- Acetal
- Carboxylic acid derivative
- Secondary amine
- Enamine
- Azacycle
- Secondary aliphatic amine
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Amine
- Organooxygen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organohalogen compound
- Organochloride
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-006x-9005200000-98df3b31c9d7a0ea8c9b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0059-0015900000-5d813bb035601f752e65 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-000l-0019000000-3e3b6549a7821bc27951 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-059i-9008100000-153ee29cfcb6dfa77efc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dr-9046000000-218219f66c08cdfbbf77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00fu-8193000000-b3a1e799ddf3fccfe407 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-9005300000-55fb7a320bd4e785d686 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fri-8019000000-f6a29f367280ef5a5809 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9038000000-73d4443b6ba434fe955f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0009300000-d9dc6ba0a22d25f76bb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0239000000-4d5f4e7491bf6796e5a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-4394000000-13e58fdf70cf215717c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009100000-f5a663becfb61e994b0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9004000000-affe81c5e27270212cc7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9021000000-6372b017c8ce4be6be65 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04920 |
|---|
| HMDB ID | HMDB0250321 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Clevidipine |
|---|
| Chemspider ID | 135722 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|