| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:58:43 UTC |
|---|
| Update Date | 2016-11-09 01:16:10 UTC |
|---|
| Accession Number | CHEM020284 |
|---|
| Identification |
|---|
| Common Name | Ximelagatran |
|---|
| Class | Small Molecule |
|---|
| Description | Ximelagatran is an anticoagulant intended to become a replacement for warfarin by overcoming the dietary restrictions, drug interaction, and monitoring issues associated with the former. In 2006, its manufacturer AstraZeneca announced that it would not attempt to market ximelagatran after reports of hepatotoxicity (liver damage) during trials, and to discontinue its distribution in countries where the drug had been approved. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
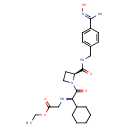
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ethyl 2-[[(1R)-1-cyclohexyl-2- [(2S)-2-[[4-(n'-hydroxycarbamimidoyl) phenyl]methylcarbamoyl]azetidin-1-yl]- 2-oxo-ethyl]amino]acetate | ChEBI | | Exanta | ChEBI | | Exarta | ChEBI | | H 376-95 | ChEBI | | H 376/95 | ChEBI | | H 37695 | ChEBI | | Ximelagatran (oxime form) | ChEBI | | Ximelagatranum | ChEBI | | Ethyl 2-[[(1R)-1-cyclohexyl-2- [(2S)-2-[[4-(n'-hydroxycarbamimidoyl) phenyl]methylcarbamoyl]azetidin-1-yl]- 2-oxo-ethyl]amino]acetic acid | Generator | | XI-melagatran | HMDB | | Glycine, N-((1R)1-cyclohexyl-2-((2S)-((((4-(amino(hydroxyimino)methyl)phenyl)methyl)amino)carbonyl)-1-azetidinyl)2-oxoethyl)-ethyl ester | HMDB | | Glycine, N-((1)1-cyclohexyl-2-((2)-((((4-(amino(hydroxyimino)methyl)phenyl)methyl)amino)carbonyl)-1-azetidinyl)2-oxoethyl)-ethyl ester | HMDB |
|
|---|
| Chemical Formula | C24H35N5O5 |
|---|
| Average Molecular Mass | 473.565 g/mol |
|---|
| Monoisotopic Mass | 473.264 g/mol |
|---|
| CAS Registry Number | 192939-46-1 |
|---|
| IUPAC Name | ethyl 2-{[(1R)-1-cyclohexyl-2-[(2S)-2-[({4-[(Z)-N'-hydroxycarbamimidoyl]phenyl}methyl)carbamoyl]azetidin-1-yl]-2-oxoethyl]amino}acetate |
|---|
| Traditional Name | ximelagatran |
|---|
| SMILES | CCOC(=O)CN[C@@H](C(=O)N1CC[C@H]1C(=O)NCC1=CC=C(C=C1)C(\N)=N\O)C1CCCCC1 |
|---|
| InChI Identifier | InChI=1S/C24H35N5O5/c1-2-34-20(30)15-26-21(17-6-4-3-5-7-17)24(32)29-13-12-19(29)23(31)27-14-16-8-10-18(11-9-16)22(25)28-33/h8-11,17,19,21,26,33H,2-7,12-15H2,1H3,(H2,25,28)(H,27,31)/t19-,21+/m0/s1 |
|---|
| InChI Key | ZXIBCJHYVWYIKI-PZJWPPBQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Alpha-amino acid ester
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- Monocyclic benzene moiety
- Benzenoid
- Amidoxime
- Tertiary carboxylic acid amide
- Amino acid or derivatives
- Azetidine
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid ester
- Amidine
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Secondary aliphatic amine
- Monocarboxylic acid or derivatives
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic nitrogen compound
- Amine
- Organic oxide
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004j-4982400000-32fe628a565ad95d34f7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03ka-0910600000-44563426df349abe783a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ot-1900000000-993c6a2f4f07b4664b53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-2900000000-7463f581ac8e0bfad363 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-010r-1091600000-caf9b25e0e59704abc3d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0i6r-1390100000-fa8587138131e92bfa4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f6t-9780000000-e48b26348d984d96c2ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0011900000-d107c431444eff177ff3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dj-0242900000-d014519f397e125b9c12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-07cs-2910000000-1bde1af9d5c933ea620b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-23339a41ce7aee480594 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0209400000-b804ae6102407413420b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2691100000-8ca0829e1959417b49c4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04898 |
|---|
| HMDB ID | HMDB0015603 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ximelagatran |
|---|
| Chemspider ID | 7848559 |
|---|
| ChEBI ID | 65172 |
|---|
| PubChem Compound ID | 656635 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Eriksson H, Wahlander K, Gustafsson D, Welin LT, Frison L, Schulman S: A randomized, controlled, dose-guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. J Thromb Haemost. 2003 Jan;1(1):41-7. | | 2. Francis CW, Berkowitz SD, Comp PC, Lieberman JR, Ginsberg JS, Paiement G, Peters GR, Roth AW, McElhattan J, Colwell CW Jr: Comparison of ximelagatran with warfarin for the prevention of venous thromboembolism after total knee replacement. N Engl J Med. 2003 Oct 30;349(18):1703-12. | | 3. Weitz JI: New anticoagulants for treatment of venous thromboembolism. Circulation. 2004 Aug 31;110(9 Suppl 1):I19-26. | | 4. Bergqvist D, Solhaug JH, Holmdahl L, Eriksson UG, Andersson M, Boberg B, Ogren M: Pharmacokinetics, preliminary efficacy and safety of subcutaneous melagatran and oral ximelagatran : a multicentre study of thromboprophylaxis in elective abdominal surgery. Clin Drug Investig. 2004;24(3):127-36. | | 5. Koscielny J, Kiesewetter H, Jorg I, Harenberg J: Ximelagatran for treatment and prophylaxis of recurrent events in deep vein thrombosis. Clin Appl Thromb Hemost. 2007 Jul;13(3):299-307. | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=12846595 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=15487959 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=16084146 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=16106594 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=16123912 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=16511607 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=16767816 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=17319469 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=17636192 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=19028773 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=20020269 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=28338626 |
|
|---|