| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:56:13 UTC |
|---|
| Update Date | 2016-11-09 01:16:09 UTC |
|---|
| Accession Number | CHEM020231 |
|---|
| Identification |
|---|
| Common Name | Normorphine |
|---|
| Class | Small Molecule |
|---|
| Description | Normorphine is an opiate analogue, the N-demethylated derivative of morphine, that was first described in the 1950s when a large group of N-substituted morphine analogues were characterized for activity. The compound has relatively little opioid activity in its own right, but is a useful intermediate which can be used to produce both opioid antagonists such as nalorphine, and also potent opioid agonists such as N-phenethylnormorphine. It is also a major metabolite of morphine, with its formation from morphine catalyzed by the liver enzymes CYP3A4 and CYP2C8.Normorphine is a controlled substance listed under the Single Convention On Narcotic Drugs 1961 and the laws in various states implementing it; for example, in the United States it is a Schedule I Narcotic controlled substance, with an ACSCN of 9313 and an annual aggregate manufacturing quota of 18 grammes in 2014, unchanged from the prior year. The salts in use are the free base hexahydrate (free base conversion ratio 0.715), and hydrochloride (0.833). |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
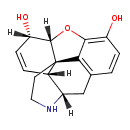
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Normorphine | MeSH | | (5alpha,6alpha)-7,8-Didehydro-4,5-epoxymorphinan-3,6-diol hydrochloride | MeSH | | N-Demethylmorphine | MeSH | | 4,5-Epoxy-3,6-dihydroxymorphin-7-ene | MeSH | | Normorphine sulfamate | MeSH | | Normorphine hydrochloride | MeSH | | Desmethylmorphine | MeSH | | Normorphine perchlorate | MeSH | | Normorphine | MeSH |
|
|---|
| Chemical Formula | C16H17NO3 |
|---|
| Average Molecular Mass | 271.316 g/mol |
|---|
| Monoisotopic Mass | 271.121 g/mol |
|---|
| CAS Registry Number | 466-97-7 |
|---|
| IUPAC Name | (1S,5S,13R,14S,17R)-12-oxa-4-azapentacyclo[9.6.1.0¹,¹³.0⁵,¹⁷.0⁷,¹⁸]octadeca-7(18),8,10,15-tetraene-10,14-diol |
|---|
| Traditional Name | (1S,5S,13R,14S,17R)-12-oxa-4-azapentacyclo[9.6.1.0¹,¹³.0⁵,¹⁷.0⁷,¹⁸]octadeca-7(18),8,10,15-tetraene-10,14-diol |
|---|
| SMILES | [H][C@@]12OC3=C(O)C=CC4=C3[C@@]11CCN[C@@]([H])(C4)[C@]1([H])C=C[C@]2([H])O |
|---|
| InChI Identifier | InChI=1S/C16H17NO3/c18-11-3-1-8-7-10-9-2-4-12(19)15-16(9,5-6-17-10)13(8)14(11)20-15/h1-4,9-10,12,15,17-19H,5-7H2/t9-,10-,12-,15-,16-/m0/s1 |
|---|
| InChI Key | ONBWJWYUHXVEJS-WYCAWQEYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as morphinans. These are polycyclic compounds with a four-ring skeleton with three condensed six-member rings forming a partially hydrogenated phenanthrene moiety, one of which is aromatic while the two others are alicyclic. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Morphinans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Morphinans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Morphinan
- Phenanthrene
- Tetralin
- Coumaran
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Piperidine
- Benzenoid
- Secondary alcohol
- Secondary aliphatic amine
- Ether
- Oxacycle
- Secondary amine
- Azacycle
- Organoheterocyclic compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Amine
- Alcohol
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-f04dca2e66d95b31aa25 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0090000000-467a6bcd8ff91cbefd44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03l1-1690000000-971300dede3c8e5c9094 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-1a3f0fd2eeb491719460 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-5ff44da00a74913c4409 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03l0-0590000000-00069e84198f236ab6bc | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Normorphine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 443403 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|