| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:55:21 UTC |
|---|
| Update Date | 2016-11-09 01:16:09 UTC |
|---|
| Accession Number | CHEM020212 |
|---|
| Identification |
|---|
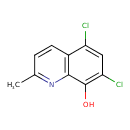
| Common Name | 5,7-Dichloro-8-hydroxy-2-methylquinoline |
|---|
| Class | Small Molecule |
|---|
| Description | A monohydroxyquinoline that is quinolin-8-ol which is substituted by a methyl group at position 2 and by chlorine at positions 5 and 7. An antifungal and antibacterial, it was formerly used for topical treatment of skin conditions and vaginal infections. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Methyl-5,7-dichloro-8-hydroxyquinoline | ChEBI | | 5,7-Dichloro-2-methyl-8-hydroxyquinoline | ChEBI | | 5,7-Dichloro-2-methyl-8-quinolinol | ChEBI | | 5,7-Dichloro-8-hydroxy-2-methylquinoline | ChEBI | | 5,7-Dichloro-8-hydroxyquinaldine | ChEBI | | 5,7-Dichloro-8-quinaldinol | ChEBI | | Chlorquinaldolum | ChEBI | | Clorquinaldol | ChEBI | | 5,7 Dichloro 2 methyl 8 quinolinol | MeSH | | Afungil | MeSH | | Chlorchinaldine | MeSH | | Chlorchinaldol | MeSH | | Chloroquinaldol | MeSH | | Sterosan | MeSH |
|
|---|
| Chemical Formula | C10H7Cl2NO |
|---|
| Average Molecular Mass | 228.070 g/mol |
|---|
| Monoisotopic Mass | 226.990 g/mol |
|---|
| CAS Registry Number | 72-80-0 |
|---|
| IUPAC Name | 5,7-dichloro-2-methylquinolin-8-ol |
|---|
| Traditional Name | chlorquinaldol |
|---|
| SMILES | CC1=NC2=C(C=C1)C(Cl)=CC(Cl)=C2O |
|---|
| InChI Identifier | InChI=1S/C10H7Cl2NO/c1-5-2-3-6-7(11)4-8(12)10(14)9(6)13-5/h2-4,14H,1H3 |
|---|
| InChI Key | GPTXWRGISTZRIO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as chloroquinolines. Chloroquinolines are compounds containing a quinoline moiety, which carries one or more chlorine atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Haloquinolines |
|---|
| Direct Parent | Chloroquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Chloroquinoline
- 8-hydroxyquinoline
- Methylpyridine
- Aryl chloride
- Aryl halide
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004v-1930000000-05617bea58b154708441 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-0b8337dd00afa205fb55 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0090000000-939be2b48f6ac7189a2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0920000000-c1fb0871b2725cb1e7dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-87f1616b9635b54c0112 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0190000000-be8b97613dafc25837bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0900000000-1a43f8fc0417fc208765 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-d83ab1ef8f7fc3a8bcac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0090000000-d83ab1ef8f7fc3a8bcac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06sc-3900000000-fdd347809f32520bc490 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-077822917bcd2e3e63be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0090000000-077822917bcd2e3e63be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-9500000000-bffdc8f00135bca23644 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13306 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Chlorquinaldol |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 74500 |
|---|
| PubChem Compound ID | 6301 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|