| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:52:13 UTC |
|---|
| Update Date | 2016-11-09 01:16:09 UTC |
|---|
| Accession Number | CHEM020171 |
|---|
| Identification |
|---|
| Common Name | Clobetasol |
|---|
| Class | Small Molecule |
|---|
| Description | A 3-oxo-Delta(1),Delta(4)-steroid that is 16beta-methylpregna-1,4-diene-3,20-dione bearing hydroxy groups at the 11beta and 17alpha positions, fluorine at position 9, and a chlorine substituent at position 21. It is used as its 17alpha-propionate ester to treat various skin disorders, including exzema and psoriasis. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
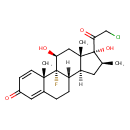
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (11beta,16beta)-21-Chloro-9-fluoro-11,17-dihydroxy-16-methylpregna-1,4-diene-3,20-dione | ChEBI | | Clobetasolum | ChEBI | | Clobecort amex | Kegg | | (11b,16b)-21-Chloro-9-fluoro-11,17-dihydroxy-16-methylpregna-1,4-diene-3,20-dione | Generator | | (11Β,16β)-21-chloro-9-fluoro-11,17-dihydroxy-16-methylpregna-1,4-diene-3,20-dione | Generator | | Clobetasol 17 propionate | HMDB | | Clobetasol 17-propionate | HMDB | | Clobetasol propionate | HMDB | | Clobex | HMDB | | Clofenazon | HMDB | | Cormax | HMDB | | Dermovate | HMDB | | Embeline | HMDB | | Embeline e | HMDB | | OLUX | HMDB | | Temovate | HMDB |
|
|---|
| Chemical Formula | C22H28ClFO4 |
|---|
| Average Molecular Mass | 410.907 g/mol |
|---|
| Monoisotopic Mass | 410.166 g/mol |
|---|
| CAS Registry Number | 25122-41-2 |
|---|
| IUPAC Name | (1R,2S,10S,11S,13S,14R,15S,17S)-14-(2-chloroacetyl)-1-fluoro-14,17-dihydroxy-2,13,15-trimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-3,6-dien-5-one |
|---|
| Traditional Name | clobetasol |
|---|
| SMILES | [H][C@@]12C[C@H](C)[C@](O)(C(=O)CCl)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |
|---|
| InChI Identifier | InChI=1S/C22H28ClFO4/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,24)17(26)10-20(16,3)22(12,28)18(27)11-23/h6-7,9,12,15-17,26,28H,4-5,8,10-11H2,1-3H3/t12-,15-,16-,17-,19-,20-,21-,22-/m0/s1 |
|---|
| InChI Key | FCSHDIVRCWTZOX-DVTGEIKXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- Hydroxysteroid
- Halo-steroid
- 9-halo-steroid
- Oxosteroid
- 11-beta-hydroxysteroid
- 11-hydroxysteroid
- 3-oxo-delta-1,4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Delta-1,4-steroid
- Alpha-haloketone
- Cyclic alcohol
- Alpha-hydroxy ketone
- Tertiary alcohol
- Alpha-chloroketone
- Cyclic ketone
- Secondary alcohol
- Fluorohydrin
- Halohydrin
- Ketone
- Organooxygen compound
- Alcohol
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alkyl halide
- Alkyl fluoride
- Organofluoride
- Organochloride
- Organohalogen compound
- Alkyl chloride
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05ur-1922000000-1a2dff27fd4359dcf1ff | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-000l-3545390000-45a386f33671c09db1ef | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-00dj-0960000000-a22cd2cf411698d4cb20 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-01ox-0009400000-e9436fb9bac8eda3bf10 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-00dj-0960000000-6443ffdb9b79ef387dc3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-01w1-0692000000-4a54671d4895c5118cc1 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-01w1-0692000000-ee83dc8cb91bcf54300f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-022a-0890000000-38b8d519ad2a9ba13b3d | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0469000000-31fce0ad107cfd522ffc | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-01ox-0009400000-8d3f1a9dbe178961f1a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03ec-0009300000-eabf4bbbde18aa470559 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06vl-0139100000-5851c4786f4f11dac8a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0i6r-0396000000-ce80f2ae92c03b288ae7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0005900000-08f019701c8687b3adfa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fr-1009200000-83699a43939ad9dd09a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gc0-1029000000-d3f4258d8f19313ca5a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0009700000-1851cdf5907155e33c20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-0869400000-ff98ebd4c04b608835cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4u-2891000000-106ece766a3163e1226b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ab9-0009800000-d247e43fa37d759961e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1009000000-d289c7e30bdafdb38a90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-1019000000-0e4b816a146275aa7238 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB11750 |
|---|
| HMDB ID | HMDB0015148 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Clobetasol propionate |
|---|
| Chemspider ID | 4470588 |
|---|
| ChEBI ID | 205919 |
|---|
| PubChem Compound ID | 5311051 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|