| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:51:56 UTC |
|---|
| Update Date | 2016-11-09 01:16:08 UTC |
|---|
| Accession Number | CHEM020168 |
|---|
| Identification |
|---|
| Common Name | Terlipressin |
|---|
| Class | Small Molecule |
|---|
| Description | Terlipressin is an analogue of vasopressin used as a vasoactive drug in the management of hypotension which has been found to be effective when norepinephrine fails. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
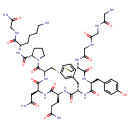
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-(N-(N-Glycylglycyl)glycyl)-8-L-lysinevasopressin | HMDB | | Glycylpressin | HMDB | | Gly-gly-gly-8-lys-vasopressin | HMDB | | Triglycylvasopressin | HMDB | | N-(alpha)-Glycyl-glycyl-glycyl-8-lysine vasopressin | HMDB | | Remestyp | HMDB | | TGLVP | HMDB | | Glypressin | HMDB | | Triglycyl lysine vasopressin | HMDB | | Glipressin | HMDB | | Terlypressin | HMDB | | Triglycyl-(8-lysine)vasopressin | HMDB | | (2S)-6-Amino-2-({[(2S)-1-[(4R,7S,10S,13S,16S,19R)-19-{[2-({2-[(2-amino-1-hydroxyethylidene)amino]-1-hydroxyethylidene}amino)-1-hydroxyethylidene]amino}-13-benzyl-6,9,12,15,18-pentahydroxy-10-[2-(C-hydroxycarbonimidoyl)ethyl]-7-[(C-hydroxycarbonimidoyl)methyl]-16-[(4-hydroxyphenyl)methyl]-1,2-dithia-5,8,11,14,17-pentaazacycloicosa-5,8,11,14,17-pentaene-4-carbonyl]pyrrolidin-2-yl](hydroxy)methylidene}amino)-N-[(C-hydroxycarbonimidoyl)methyl]hexanimidate | HMDB | | Gly gly gly 8 lys vasopressin | HMDB | | Tri-gly-8-lys- vasopressin | HMDB | | Terlipressin | MeSH |
|
|---|
| Chemical Formula | C52H74N16O15S2 |
|---|
| Average Molecular Mass | 1227.372 g/mol |
|---|
| Monoisotopic Mass | 1226.496 g/mol |
|---|
| CAS Registry Number | 14636-12-5 |
|---|
| IUPAC Name | (2S)-6-amino-2-{[(2S)-1-[(4R,7S,10S,13S,16S,19R)-19-{2-[2-(2-aminoacetamido)acetamido]acetamido}-13-benzyl-10-(2-carbamoylethyl)-7-(carbamoylmethyl)-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carbonyl]pyrrolidin-2-yl]formamido}-N-(carbamoylmethyl)hexanamide |
|---|
| Traditional Name | terlipressin |
|---|
| SMILES | NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CSSC[C@H](NC(=O)CNC(=O)CNC(=O)CN)C(=O)N[C@@H](CC2=CC=C(O)C=C2)C(=O)N[C@@H](CC2=CC=CC=C2)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)NCC(N)=O |
|---|
| InChI Identifier | InChI=1S/C52H74N16O15S2/c53-17-5-4-9-31(45(76)60-23-41(57)72)63-51(82)38-10-6-18-68(38)52(83)37-27-85-84-26-36(61-44(75)25-59-43(74)24-58-42(73)22-54)50(81)65-34(20-29-11-13-30(69)14-12-29)48(79)64-33(19-28-7-2-1-3-8-28)47(78)62-32(15-16-39(55)70)46(77)66-35(21-40(56)71)49(80)67-37/h1-3,7-8,11-14,31-38,69H,4-6,9-10,15-27,53-54H2,(H2,55,70)(H2,56,71)(H2,57,72)(H,58,73)(H,59,74)(H,60,76)(H,61,75)(H,62,78)(H,63,82)(H,64,79)(H,65,81)(H,66,77)(H,67,80)/t31-,32-,33-,34-,35-,36-,37-,38-/m0/s1 |
|---|
| InChI Key | BENFXAYNYRLAIU-QSVFAHTRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as polypeptides. These are peptides containing ten or more amino acid residues. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic Polymers |
|---|
| Class | Polypeptides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Polypeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polypeptide
- Cyclic alpha peptide
- Proline or derivatives
- Macrolactam
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Benzenoid
- Pyrrolidine
- Tertiary carboxylic acid amide
- Secondary carboxylic acid amide
- Organic disulfide
- Amino acid or derivatives
- Primary carboxylic acid amide
- Carboxamide group
- Lactam
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid derivative
- Primary amine
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary aliphatic amine
- Carbonyl group
- Organic nitrogen compound
- Organopnictogen compound
- Amine
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000l-9730000000-a8d68dda1eff985cd914 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-9510000000-891a33e18235006eee81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9100000000-e04b89517b6a7732b095 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-056r-3890000000-9b92091320e40161e6da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zml-6970000001-861b801a7476e9e5937d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fdo-9630000010-73ee99640a78fc6019c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-6590000000-47d9bd21e8229f357439 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bvi-9510000000-17fabccd2717c964fced | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0600-9400000000-66b70375d23a49bfc543 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00b9-5970000000-829ad70af249c53ff63b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-074l-9110000000-b9faa13362605fe7add7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-e33d93f0810127e18002 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB02638 |
|---|
| HMDB ID | HMDB0015569 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Terlipressin |
|---|
| Chemspider ID | 65067 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 72081 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Kam PC, Williams S, Yoong FF: Vasopressin and terlipressin: pharmacology and its clinical relevance. Anaesthesia. 2004 Oct;59(10):993-1001. | | 2. Matok I, Vard A, Efrati O, Rubinshtein M, Vishne T, Leibovitch L, Adam M, Barzilay Z, Paret G: Terlipressin as rescue therapy for intractable hypotension due to septic shock in children. Shock. 2005 Apr;23(4):305-10. | | 3. Leone M, Charvet A, Delmas A, Albanese J, Martin C, Boyle WA: Terlipressin: a new therapeutic for calcium-channel blockers overdose. J Crit Care. 2005 Mar;20(1):114-5. | | 4. Klein M, Weksler N, Borer A, Koyfman L, Kesslin J, Gurman GM: Terlipressin facilitates transport of septic patients treated with norepinephrine. Isr Med Assoc J. 2006 Oct;8(10):691-3. | | 5. Pesaturo AB, Jennings HR, Voils SA: Terlipressin: vasopressin analog and novel drug for septic shock. Ann Pharmacother. 2006 Dec;40(12):2170-7. Epub 2006 Dec 5. |
|
|---|