| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:50:36 UTC |
|---|
| Update Date | 2016-11-09 01:16:08 UTC |
|---|
| Accession Number | CHEM020144 |
|---|
| Identification |
|---|
| Common Name | Alfentanil hydrochloride |
|---|
| Class | Small Molecule |
|---|
| Description | Alfentanil (R-39209, trade name Alfenta, Rapifen in Australia) is a potent but short-acting synthetic opioid analgesic drug, used for anaesthesia in surgery. It is an analogue of fentanyl with around 1/4 to 1/10 the potency of fentanyl and around 1/3 of the duration of action, but with an onset of effects 4x faster than fentanyl. Alfentanil has a pKa of approximately 6.5, which leads to a very high proportion of the drug being uncharged at physiologic pH. This unique characteristic is responsible for its rapid onset. It is an agonist at mu opioid receptors.
While alfentanil tends to cause fewer cardiovascular complications than other similar drugs such as fentanyl and remifentanil, it tends to give stronger respiratory depression and so requires careful monitoring of breathing and vital signs. Almost exclusively used by anesthesia providers during portions of a case where quick, fast acting (though not long lasting) pain control is needed (i.e. during a nerve block, head pinning etc..) Alfentanil is administered by the parenteral (injected) route for fast onset of effects and precise control of dosage.
Alfentanil is a restricted drug which is classified as Schedule II in the US, according to the U.S. DEA website.Alfentanil was discovered at Janssen Pharmaceutica in 1976.
Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
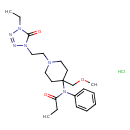
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Limifen | MeSH | | Alfentanil | MeSH | | Alfenta | MeSH | | Rapifen | MeSH | | Fanaxal | MeSH | | Alfentanyl | MeSH | | Alfentanil HCL | ChEMBL | | Alfentanil hydrochloride | MeSH | | Esteve brand OF alfentanil hydrochloride | MeSH | | Janssen brand OF alfentanil hydrochloride | MeSH | | ICI brand OF alfentanil hydrochloride | MeSH |
|
|---|
| Chemical Formula | C21H33ClN6O3 |
|---|
| Average Molecular Mass | 452.980 g/mol |
|---|
| Monoisotopic Mass | 452.230 g/mol |
|---|
| CAS Registry Number | 69049-06-5 |
|---|
| IUPAC Name | N-{1-[2-(4-ethyl-5-oxo-4,5-dihydro-1H-1,2,3,4-tetrazol-1-yl)ethyl]-4-(methoxymethyl)piperidin-4-yl}-N-phenylpropanamide hydrochloride |
|---|
| Traditional Name | alfentanil hydrochloride |
|---|
| SMILES | Cl.CCN1N=NN(CCN2CCC(COC)(CC2)N(C(=O)CC)C2=CC=CC=C2)C1=O |
|---|
| InChI Identifier | InChI=1S/C21H32N6O3.ClH/c1-4-19(28)27(18-9-7-6-8-10-18)21(17-30-3)11-13-24(14-12-21)15-16-26-20(29)25(5-2)22-23-26;/h6-10H,4-5,11-17H2,1-3H3;1H |
|---|
| InChI Key | AQORHZJDCHLLJN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anilides. These are organic heterocyclic compounds derived from oxoacids RkE(=O)l(OH)m (l not 0) by replacing an OH group by the NHPh group or derivative formed by ring substitution. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Anilides |
|---|
| Direct Parent | Anilides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anilide
- Piperidine
- Azole
- Heteroaromatic compound
- Tertiary carboxylic acid amide
- Tetrazole
- Amino acid or derivatives
- Carboxamide group
- Tertiary amine
- Tertiary aliphatic amine
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Azacycle
- Organoheterocyclic compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Amine
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Hydrochloride
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000900000-1a3c5e33903698b7b5ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0000900000-1a3c5e33903698b7b5ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0000900000-1a3c5e33903698b7b5ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-8d6cb6bbf46b973b6d67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0000900000-8d6cb6bbf46b973b6d67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0000900000-8d6cb6bbf46b973b6d67 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Alfentanil |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 64761 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|