| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:43:00 UTC |
|---|
| Update Date | 2016-11-09 01:16:07 UTC |
|---|
| Accession Number | CHEM020031 |
|---|
| Identification |
|---|
| Common Name | Monatepil |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
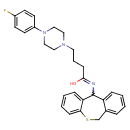
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-[4-(4-Fluorophenyl)piperazin-1-yl]-N-{9-thiatricyclo[9.4.0.0,]pentadeca-1(15),3,5,7,11,13-hexaen-2-yl}butanimidate | Generator | | Monatepil, (R)-isomer | MeSH | | 11-((4-(4-(4-Fluorophenyl)-1-piperazinyl)butyryl)amino)-6,11-dihydrodibenzo(b,e)thiepin maleate | MeSH | | Monatepil maleate | MeSH | | Monatepil | MeSH | | Monatepil X maleate | MeSH | | Monatepil, (+-) monatepil | MeSH | | Monatepil, (S)-isomer | MeSH | | 4-[4-(4-Fluorophenyl)piperazin-1-yl]-N-{9-thiatricyclo[9.4.0.0³,⁸]pentadeca-1(15),3,5,7,11,13-hexaen-2-yl}butanimidate | Generator |
|
|---|
| Chemical Formula | C28H30FN3OS |
|---|
| Average Molecular Mass | 475.630 g/mol |
|---|
| Monoisotopic Mass | 475.209 g/mol |
|---|
| CAS Registry Number | 103377-41-9 |
|---|
| IUPAC Name | 4-[4-(4-fluorophenyl)piperazin-1-yl]-N-{9-thiatricyclo[9.4.0.0³,⁸]pentadeca-1(15),3,5,7,11,13-hexaen-2-yl}butanimidic acid |
|---|
| Traditional Name | 4-[4-(4-fluorophenyl)piperazin-1-yl]-N-{9-thiatricyclo[9.4.0.0³,⁸]pentadeca-1(15),3,5,7,11,13-hexaen-2-yl}butanimidic acid |
|---|
| SMILES | OC(CCCN1CCN(CC1)C1=CC=C(F)C=C1)=NC1C2=CC=CC=C2CSC2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C28H30FN3OS/c29-22-11-13-23(14-12-22)32-18-16-31(17-19-32)15-5-10-27(33)30-28-24-7-2-1-6-21(24)20-34-26-9-4-3-8-25(26)28/h1-4,6-9,11-14,28H,5,10,15-20H2,(H,30,33) |
|---|
| InChI Key | WFNRNNUZFPVBSM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dibenzothiepins. Dibenzothiepins are compounds containing a dibenzothiepin moiety, which consists of two benzene connected by a thiepine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzothiepins |
|---|
| Sub Class | Dibenzothiepins |
|---|
| Direct Parent | Dibenzothiepins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dibenzothiepin
- Phenylpiperazine
- N-arylpiperazine
- Aryl thioether
- Tertiary aliphatic/aromatic amine
- Dialkylarylamine
- Aniline or substituted anilines
- Halobenzene
- Fluorobenzene
- N-alkylpiperazine
- Alkylarylthioether
- N-acyl-amine
- Aryl fluoride
- Fatty acyl
- Fatty amide
- 1,4-diazinane
- Benzenoid
- Monocyclic benzene moiety
- Aryl halide
- Piperazine
- Carboxamide group
- Tertiary aliphatic amine
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Tertiary amine
- Azacycle
- Thioether
- Carboxylic acid derivative
- Organooxygen compound
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organopnictogen compound
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06xy-2690100000-c6c5ce4558bff7170b0b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090600000-c365b6533464eb0dea73 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0190000000-ff5e859b798d9af3b469 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-1790000000-959a53a5e210d0cb7446 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0020900000-222850a9cff9dd13e22a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00fs-0090700000-2666ee79dbfee77e0d5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00b9-2970000000-b4aeb984d911c3d4d01c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0010900000-d5161909eea3ed0365b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-0090300000-dba48f2862faff1a295b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0491200000-aa7d28540ae2e2364358 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-5498382d811ceaae3939 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0051900000-54dfdfe992ff89f7d59f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dr-0940000000-9593405390a65d7f28d7 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 60810 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|