| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:40:40 UTC |
|---|
| Update Date | 2016-11-09 01:16:06 UTC |
|---|
| Accession Number | CHEM019985 |
|---|
| Identification |
|---|
| Common Name | Bardoxolone methyl |
|---|
| Class | Small Molecule |
|---|
| Description | Bardoxolone methyl (also known as “RTA 402”, “CDDO-methyl ester”, and CDDO-Me) is an experimental and orally-bioavailable semi-synthetic triterpenoid, based on the scaffold of the natural product oleanolic acid. Pre-clinical studies indicate that the compound acts as an activator of the Nrf2 pathway and an inhibitor of the NF-κB pathway. A phase 3 clinical trial evaluating bardoxolone methyl for the treatment of chronic kidney disease (CKD) was terminated in October 2012 after patients treated with the drug were found to have experienced a higher rate of heart-related adverse events, including heart failure, hospitalizations, and deaths. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
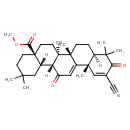
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| CDDO methyl ester | MeSH | | CDDO-me | MeSH | | Methyl 2-cyano-3,12-dioxoolean-1,9-dien-28-Oate | MeSH | | 2-Cyano-3,12-dioxoolean-1,9-dien-28-Oic acid methyl ester | MeSH | | NSC-713200cddo-Me | ChEMBL | | Methyl (4as,6ar,6BS,8ar,12as,14ar,14BS)-11-cyano-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,3,4,5,6,7,8,8a,14a,14b-decahydropicene-4a-carboxylic acid | Generator | | Bardoxolone methyl | MeSH |
|
|---|
| Chemical Formula | C32H43NO4 |
|---|
| Average Molecular Mass | 505.699 g/mol |
|---|
| Monoisotopic Mass | 505.319 g/mol |
|---|
| CAS Registry Number | 218600-53-4 |
|---|
| IUPAC Name | methyl (4aS,6aR,6bS,8aR,12aS,14aR,14bS)-11-cyano-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,12a,14,14a,14b-octadecahydropicene-4a-carboxylate |
|---|
| Traditional Name | bardoxolone methyl |
|---|
| SMILES | [H][C@@]12CC(C)(C)CC[C@@]1(CC[C@]1(C)[C@]2([H])C(=O)C=C2[C@@]1(C)CC[C@@]1([H])C(C)(C)C(=O)C(=C[C@]21C)C#N)C(=O)OC |
|---|
| InChI Identifier | InChI=1S/C32H43NO4/c1-27(2)11-13-32(26(36)37-8)14-12-31(7)24(20(32)17-27)21(34)15-23-29(5)16-19(18-33)25(35)28(3,4)22(29)9-10-30(23,31)6/h15-16,20,22,24H,9-14,17H2,1-8H3/t20-,22-,24-,29-,30+,31+,32-/m0/s1 |
|---|
| InChI Key | WPTTVJLTNAWYAO-KPOXMGGZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cyclohexenones. Cyclohexenones are compounds containing a cylohexenone moiety, which is a six-membered aliphatic ring that carries a ketone and has one endocyclic double bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Cyclohexenones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclohexenone
- Methyl ester
- Carboxylic acid ester
- Nitrile
- Carbonitrile
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000960000-886b4458bfb1ffdc232a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-059i-0110910000-be3c041d1e749a50d0eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-2012900000-a07603b0bbe84296c737 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000290000-f5c3e7219bea46cda80b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-1000790000-55603c82debbc5c40d04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4u-2001900000-d4d97d75abee285e120e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Bardoxolone methyl |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 400769 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|