| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:39:39 UTC |
|---|
| Update Date | 2016-11-09 01:16:06 UTC |
|---|
| Accession Number | CHEM019966 |
|---|
| Identification |
|---|
| Common Name | Cefditorin pivoxil |
|---|
| Class | Small Molecule |
|---|
| Description | The pivaloyloxymethyl ester prodrug of cefditoren. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
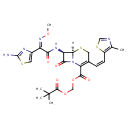
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cefditoren pivaloyloxymethyl ester | ChEBI | | Pivaloyloxymethyl (+)-(6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-[(Z)-2-(4-methyl-1,3-thiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate | ChEBI | | CDTR-PI | Kegg | | Meiact | Kegg | | Spectracef | Kegg | | Pivaloyloxymethyl (+)-(6R,7R)-7-{[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-[(Z)-2-(4-methyl-1,3-thiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | Generator | | 7-(2-(2-Aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-(2-(4-methylthiazol-5-yl)ethenyl)cephem-4-carboxylic acid pivaloyloxymethyl ester | MeSH | | (-)-(6R,7R)-2,2-Dimethylpropionyloxymethyl 7-((Z)-2-(2-aminothiazol-4-yl)-2-methoxyiminoacetamido)-3-((Z)-2-(4-methylthiazol-5-yl)ethenyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate | MeSH | | 2,2-Dimethylpropanoyloxymethyl (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-[(Z)-2-(4-methyl-1,3-thiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | Generator | | Cefditoren pivoxil | MeSH |
|
|---|
| Chemical Formula | C25H28N6O7S3 |
|---|
| Average Molecular Mass | 620.710 g/mol |
|---|
| Monoisotopic Mass | 620.118 g/mol |
|---|
| CAS Registry Number | 117467-28-4 |
|---|
| IUPAC Name | [(6R,7R)-7-[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamido]-3-[(Z)-2-(4-methyl-1,3-thiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carbonyloxy]methyl 2,2-dimethylpropanoate |
|---|
| Traditional Name | spectracef |
|---|
| SMILES | [H][C@]12SCC(\C=C/C3=C(C)N=CS3)=C(N1C(=O)[C@H]2NC(=O)C(=N/OC)\C1=CSC(N)=N1)C(=O)OCOC(=O)C(C)(C)C |
|---|
| InChI Identifier | InChI=1S/C25H28N6O7S3/c1-12-15(41-10-27-12)7-6-13-8-39-21-17(29-19(32)16(30-36-5)14-9-40-24(26)28-14)20(33)31(21)18(13)22(34)37-11-38-23(35)25(2,3)4/h6-7,9-10,17,21H,8,11H2,1-5H3,(H2,26,28)(H,29,32)/b7-6-,30-16-/t17-,21-/m1/s1 |
|---|
| InChI Key | AFZFFLVORLEPPO-UVYJNCLZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylpiperazines. Phenylpiperazines are compounds containing a phenylpiperazine skeleton, which consists of a piperazine bound to a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazinanes |
|---|
| Sub Class | Piperazines |
|---|
| Direct Parent | Phenylpiperazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpiperazine
- Tryptamine
- N-arylpiperazine
- 3-alkylindole
- Indole
- Indole or derivatives
- Anisole
- Tertiary aliphatic/aromatic amine
- Dialkylarylamine
- Aniline or substituted anilines
- Alkyl aryl ether
- Aralkylamine
- N-alkylpiperazine
- Benzenoid
- Monocyclic benzene moiety
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Ether
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organic chloride salt
- Organic zwitterion
- Organic salt
- Hydrocarbon derivative
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uel-4974423000-3773f6305878497dad40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pba-9354300000-990d8735825385978204 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-9211100000-86a011b1513bdb95ceb6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0290200000-6f0007cbe634a9a504c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zg0-1930110000-1e5a8a975b1d3b2b74d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9500000000-1bca62410985d786f2d4 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT001811 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cefditoren_Pivoxil |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 560555 |
|---|
| PubChem Compound ID | 6437877 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|