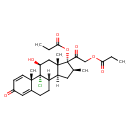
| (11beta,16beta)-9-Chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)pregna-1,4-diene-3,20-dione | ChEBI |
| 9-Chloro-11beta-hydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate | ChEBI |
| 9-Chloro-16beta-methyl-11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione 17,21-dipropionate | ChEBI |
| Aerobec | ChEBI |
| Aldecin | ChEBI |
| Anceron | ChEBI |
| Andion | ChEBI |
| Beclacin | ChEBI |
| Becloforte | ChEBI |
| Beclomet | ChEBI |
| Beclometasone 17,21-dipropionate | ChEBI |
| Beclorhinol | ChEBI |
| Becloval | ChEBI |
| Beclovent | ChEBI |
| Becodisks | ChEBI |
| Beconase | ChEBI |
| Beconasol | ChEBI |
| Becotide | ChEBI |
| Clenil-a | ChEBI |
| Entyderma | ChEBI |
| Inalone | ChEBI |
| Korbutone | ChEBI |
| Propaderm | ChEBI |
| Rino-clenil | ChEBI |
| Sanasthmax | ChEBI |
| Sanasthmyl | ChEBI |
| Vancenase | ChEBI |
| Vanceril | ChEBI |
| Viarex | ChEBI |
| Viarox | ChEBI |
| Qnasl | Kegg |
| (11b,16b)-9-Chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)pregna-1,4-diene-3,20-dione | Generator |
| (11Β,16β)-9-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)pregna-1,4-diene-3,20-dione | Generator |
| 9-Chloro-11b-hydroxy-16b-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate | Generator |
| 9-Chloro-11b-hydroxy-16b-methylpregna-1,4-diene-3,20-dione 17,21-dipropionic acid | Generator |
| 9-Chloro-11beta-hydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-dipropionic acid | Generator |
| 9-Chloro-11β-hydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate | Generator |
| 9-Chloro-11β-hydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-dipropionic acid | Generator |
| 9-Chloro-16b-methyl-11b,17,21-trihydroxypregna-1,4-diene-3,20-dione 17,21-dipropionate | Generator |
| 9-Chloro-16b-methyl-11b,17,21-trihydroxypregna-1,4-diene-3,20-dione 17,21-dipropionic acid | Generator |
| 9-Chloro-16beta-methyl-11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione 17,21-dipropionic acid | Generator |
| 9-Chloro-16β-methyl-11β,17,21-trihydroxypregna-1,4-diene-3,20-dione 17,21-dipropionate | Generator |
| 9-Chloro-16β-methyl-11β,17,21-trihydroxypregna-1,4-diene-3,20-dione 17,21-dipropionic acid | Generator |
| Beclometasone 17,21-dipropionic acid | Generator |
| Beclometasone dipropionic acid | Generator |
| Beclomethasone dipropionate | HMDB |
| 3m Brand OF beclomethasone dipropionate | HMDB |
| Asche brand OF beclomethasone dipropionate | HMDB |
| Asmabec clickhaler | HMDB |
| Beclocort | HMDB |
| Medeva brand OF beclomethasone dipropionate | HMDB |
| Orion brand OF beclomethasone dipropionate | HMDB |
| AeroBec forte | HMDB |
| Allen and hanburys brand OF beclomethasone dipropionate | HMDB |
| Apotex brand OF beclomethasone dipropionate | HMDB |
| Ascocortonyl | HMDB |
| Beclamet | HMDB |
| Beclo azu | HMDB |
| Becloturmant | HMDB |
| Filair | HMDB |
| Norton brand OF beclomethasone dipropionate | HMDB |
| Prolair | HMDB |
| Schering-plough brand OF beclomethasone dipropionate | HMDB |
| Aldo brand OF beclomethasone dipropionate | HMDB |
| Azupharma brand OF beclomethasone dipropionate | HMDB |
| Beclo asma | HMDB |
| Beclometasone | HMDB |
| Beclomethasone | HMDB |
| Beconase aq | HMDB |
| Dipropionate, beclomethasone | HMDB |
| Filair forte | HMDB |
| Fujisawa brand OF beclomethasone dipropionate | HMDB |
| Glaxo wellcome brand OF beclomethasone dipropionate | HMDB |
| GlaxoSmithKline brand 1 OF beclomethasone dipropionate | HMDB |
| GlaxoSmithKline brand 3 OF beclomethasone dipropionate | HMDB |
| Junik | HMDB |
| Nasobec aqueous | HMDB |
| Respocort | HMDB |
| Shire brand OF beclomethasone dipropionate | HMDB |
| Becodisk | HMDB |
| Qvar | HMDB |
| Schering brand 1 OF beclomethasone dipropionate | HMDB |
| United drug brand OF beclomethasone dipropionate | HMDB |
| a And H brand OF beclomethasone dipropionate | HMDB |
| Apo-beclomethasone | HMDB |
| Beclazone | HMDB |
| Beclazone easy breathe | HMDB |
| Bemedrex easyhaler | HMDB |
| Bronchocort | HMDB |
| Celltech brand OF beclomethasone dipropionate | HMDB |
| Ecobec | HMDB |
| GlaxoSmithKline brand 2 OF beclomethasone dipropionate | HMDB |
| Ivax brand OF beclomethasone dipropionate | HMDB |
| Schering brand 2 OF beclomethasone dipropionate | HMDB |
| Ventolair | HMDB |
| Viarin | HMDB |
| Beclomethasone dipropionic acid | HMDB |
| Beclometasone dipropionate | ChEBI |