| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:36:38 UTC |
|---|
| Update Date | 2016-11-09 01:16:05 UTC |
|---|
| Accession Number | CHEM019897 |
|---|
| Identification |
|---|
| Common Name | Gadoteridol |
|---|
| Class | Small Molecule |
|---|
| Description | Gadoteridol provides contrast enhancement of the brain, spine and surrounding tissues resulting in improved visualization (compared with unenhanced MRI) of lesions with abnormal vascularity or those thought to cause a disruption of the normal blood brain barrier. Gadoteridol can also be used for whole body contrast enhanced MRI including the head, neck, liver, breast, musculoskeletal system and soft tissue pathologies. n MRI, visualization of normal and pathological brain tissue depends in part on variations in the radiofrequency signal intensity that occur with changes in proton density, alteration of the T1, and variation in T2. When placed in a magnetic field, gadoteridol shortens the T1 relaxation time in tissues where it accumulates. Abnormal vascularity or disruption of the blood-brain barrier allows accumulation of gadoteridol in lesions such as neoplasms, abscesses, and subacute infarcts. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
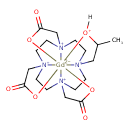
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Gadolinium 2,2',2''-[10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl]triacetate | HMDB | | Gadolinium-HP-do3a | HMDB | | Gadoteridolum | HMDB | | GD-HP-Do 3a | HMDB | | GD-HPDO3a | HMDB | | Gadolinium 2,2',2''-[10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl]triacetic acid | HMDB | | GD-HP-D03a | HMDB | | GD-Hydroxypropyl-D03a | HMDB | | GdHPDO3a | HMDB | | Gadolinium 1,4,7-triscarboxymethyl-1,4,7,10-tetraazacyclododecane | HMDB | | Gadolinium HP-do3a | HMDB | | GD-HP-DO3a | HMDB | | Prohance | HMDB | | GD(DO3a) | HMDB | | Gadolinium 1,4,7-tris(carboxymethyl)-10-(2'-hydroxypropyl)-1,4,7,10-tetraazacyclododecane | HMDB | | Gadolinium(6+) ion 2-[4,7-bis(carboxylatomethyl)-10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecan-1-yl]acetic acid | HMDB |
|
|---|
| Chemical Formula | C17H29GdN4O7 |
|---|
| Average Molecular Mass | 558.680 g/mol |
|---|
| Monoisotopic Mass | 559.128 g/mol |
|---|
| CAS Registry Number | 120066-54-8 |
|---|
| IUPAC Name | 3-methyl-16,19,24-trioxo-2,17,18,25-tetraoxa-5lambda5,8lambda5,11lambda5,14lambda5-tetraaza-1-gadolinaoctacyclo[9.6.3.3^{1,8}.2^{5,14}.0^{1,5}.0^{1,8}.0^{1,11}.0^{1,14}]pentacosan-2-ium-5,8,11,14-tetrakis(ylium)-1,1-diuide |
|---|
| Traditional Name | 3-methyl-16,19,24-trioxo-2,17,18,25-tetraoxa-5lambda5,8lambda5,11lambda5,14lambda5-tetraaza-1-gadolinaoctacyclo[9.6.3.3^{1,8}.2^{5,14}.0^{1,5}.0^{1,8}.0^{1,11}.0^{1,14}]pentacosan-2-ium-5,8,11,14-tetrakis(ylium)-1,1-diuide |
|---|
| SMILES | [Gd+3].CC(O)CN1CCN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC([O-])=O)CC1 |
|---|
| InChI Identifier | InChI=1S/C17H32N4O7.Gd/c1-14(22)10-18-2-4-19(11-15(23)24)6-8-21(13-17(27)28)9-7-20(5-3-18)12-16(25)26;/h14,22H,2-13H2,1H3,(H,23,24)(H,25,26)(H,27,28);/q;+3/p-3 |
|---|
| InChI Key | DPNNNPAKRZOSMO-UHFFFAOYSA-K |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid
- Tricarboxylic acid or derivatives
- Amino acid
- Tertiary aliphatic amine
- Tertiary amine
- Secondary alcohol
- Carboxylic acid salt
- 1,2-aminoalcohol
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Alcohol
- Organic cation
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0014735 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Gadoteridol |
|---|
| Chemspider ID | 54719 |
|---|
| ChEBI ID | 31643 |
|---|
| PubChem Compound ID | 60714 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|