| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:35:51 UTC |
|---|
| Update Date | 2016-11-09 01:16:05 UTC |
|---|
| Accession Number | CHEM019869 |
|---|
| Identification |
|---|
| Common Name | Cilazapril |
|---|
| Class | Small Molecule |
|---|
| Description | Cilazapril is an ACE inhibtor class drug used in the treatment of hypertension and heart failure. It belongs to the angiotensin-converting enzyme inhibitors (ACE inhibitors) class of drugs. It is a prodrug that is hydrolyzed after absorption to its main metabolite cilazaprilat. It is branded as Cilazapril in Canada and other countries, Cilazapril and Cilazapril in a number of European countries, among many other names. None of these varieties are available in the United States. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
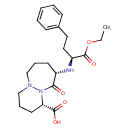
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cilazapril anhydrous | ChEBI | | Cilazaprilum | ChEBI | | Dynorm | ChEBI | | Inhibace | ChEBI | | Ro 34-2848 | ChEBI | | Vascace | ChEBI | | Vascase | ChEBI | | Monohydrate, cilazapril | HMDB | | Cilazapril monohydrate | HMDB | | Cilazapril, (s*)-isomer | HMDB | | Hydrate, cilazapril | HMDB | | Anhydrous, cilazapril | HMDB | | Cilazapril hydrate | HMDB | | Cilazapril monohydrobromide | HMDB | | Cilazapril, anhydrous | HMDB | | Monohydrobromide, cilazapril | HMDB |
|
|---|
| Chemical Formula | C22H31N3O5 |
|---|
| Average Molecular Mass | 417.499 g/mol |
|---|
| Monoisotopic Mass | 417.226 g/mol |
|---|
| CAS Registry Number | 88768-40-5 |
|---|
| IUPAC Name | (1S,9S)-9-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}-10-oxo-octahydro-1H-pyridazino[1,2-a][1,2]diazepine-1-carboxylic acid |
|---|
| Traditional Name | inhibace |
|---|
| SMILES | CCOC(=O)[C@H](CCC1=CC=CC=C1)N[C@H]1CCCN2CCC[C@H](N2C1=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C22H31N3O5/c1-2-30-22(29)18(13-12-16-8-4-3-5-9-16)23-17-10-6-14-24-15-7-11-19(21(27)28)25(24)20(17)26/h3-5,8-9,17-19,23H,2,6-7,10-15H2,1H3,(H,27,28)/t17-,18-,19-/m0/s1 |
|---|
| InChI Key | HHHKFGXWKKUNCY-FHWLQOOXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Alpha-amino acid ester
- Alpha-amino acid or derivatives
- 1,2-diazepane
- Fatty acid ester
- Diazepane
- Aralkylamine
- Dicarboxylic acid or derivatives
- 1,2-diazinane
- Pyridazine
- Benzenoid
- Fatty acyl
- Monocyclic benzene moiety
- Carboxylic acid hydrazide
- Amino acid
- Amino acid or derivatives
- Carboxylic acid ester
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid
- Secondary aliphatic amine
- Secondary amine
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organopnictogen compound
- Amine
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-3119000000-cf816839d9e62aaeb51d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-014i-3100900000-8a98b7cfd4306c863f3b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0114900000-46b169656e56da84df00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00r6-3339300000-597e65efc2ccd4259fc6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-7930000000-01cd40a3307d1d9136e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0100-2609400000-2b63f9aade5f2408e90c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00fs-2029000000-ef46b50c866af838f6d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-9413000000-bf81f08b26484eff95d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0001900000-2155fa52bd310940aaa1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kf-0129300000-d64d5ab791860881b405 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0560-5901000000-8c919145e29e13e6d804 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0001900000-13fb2fadf7c261ea1f97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014l-3289700000-05092f6b096b7ae757aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0019-2982000000-4f5dadbd2ce238e96eef | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01340 |
|---|
| HMDB ID | HMDB0015433 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cilazapril |
|---|
| Chemspider ID | 50831 |
|---|
| ChEBI ID | 553655 |
|---|
| PubChem Compound ID | 56330 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|