| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:21:03 UTC |
|---|
| Update Date | 2016-11-09 01:16:02 UTC |
|---|
| Accession Number | CHEM019632 |
|---|
| Identification |
|---|
| Common Name | Mefloquine hydrochloride |
|---|
| Class | Small Molecule |
|---|
| Description | Mefloquine, sold under the brand names Lariam among others, is a medication used to prevent or treat malaria. When used for prevention it is typically started before potential exposure and continued for several weeks after potential exposure. It can be used to treat mild or moderate malaria but is not recommended for severe malaria. It is taken by mouth.Common side effects include vomiting, diarrhea, headaches, and a rash. Serious side effects include potentially long-term mental health problems such as depression, hallucinations, and anxiety and neurological side effects such as poor balance, seizures, and ringing in the ears. It is therefore not recommended in people with a history of mental health problems or epilepsy. It appears to be safe during pregnancy and breastfeeding.Mefloquine was developed by the United States Army in the 1970s and came into use in the mid 1980s. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. It is available as a generic medication. The wholesale price in the developing world is about US$0.6–1.3 per dose. In the United Kingdom it costs the NHS £1.82 per dose. In the United States it costs about $10 a dose. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
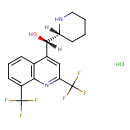
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Mephloquine | MeSH | | Lariam | MeSH | | Mefloquine | MeSH | | Roche brand OF mefloquine hydrochloride | MeSH | | Hoffmann la roche brand OF mefloquine hydrochloride | MeSH | | Hoffmann-la roche brand OF mefloquine hydrochloride | MeSH |
|
|---|
| Chemical Formula | C17H17ClF6N2O |
|---|
| Average Molecular Mass | 414.773 g/mol |
|---|
| Monoisotopic Mass | 414.093 g/mol |
|---|
| CAS Registry Number | 51773-92-3 |
|---|
| IUPAC Name | (S)-[2,8-bis(trifluoromethyl)quinolin-4-yl](2R)-piperidin-2-ylmethanol hydrochloride |
|---|
| Traditional Name | (-)-mefloquine hydrochloride |
|---|
| SMILES | Cl.[H][C@](O)(C1=CC(=NC2=C1C=CC=C2C(F)(F)F)C(F)(F)F)[C@@]1([H])CCCCN1 |
|---|
| InChI Identifier | InChI=1S/C17H16F6N2O.ClH/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11;/h3-5,8,12,15,24,26H,1-2,6-7H2;1H/t12-,15+;/m1./s1 |
|---|
| InChI Key | WESWYMRNZNDGBX-YLCXCWDSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 4-quinolinemethanols. These are organoheterocyclic compounds containing a quinoline moiety substituted at the 4-position with a methanol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | 4-quinolinemethanols |
|---|
| Direct Parent | 4-quinolinemethanols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4-quinolinemethanol
- Aralkylamine
- Piperidine
- Pyridine
- Benzenoid
- Heteroaromatic compound
- 1,2-aminoalcohol
- Secondary alcohol
- Secondary aliphatic amine
- Secondary amine
- Azacycle
- Alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Organic nitrogen compound
- Amine
- Alkyl halide
- Aromatic alcohol
- Alkyl fluoride
- Hydrochloride
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-03di-2379000000-c78ceebdc429fe31700e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000900000-825d3620fdf0522f1406 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0000900000-825d3620fdf0522f1406 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0000900000-825d3620fdf0522f1406 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-b9a319ec6eb9dd4b2d58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0000900000-b9a319ec6eb9dd4b2d58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0000900000-b9a319ec6eb9dd4b2d58 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT000311 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Mefloquine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 65329 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|