| (+)-2-(p-Bromo-alpha-(2-dimethylaminoethyl)benzyl)pyridine maleate | ChEBI |
| (+)-3-(4-Bromophenyl)-N,N-dimethyl-3-(2-pyridinyl)-1-propanamine maleate | ChEBI |
| (+)-3-(4-Bromophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine maleate | ChEBI |
| (+)-3-(p-Bromophenyl)-3-(2-pyridyl)-N,N-dimethylpropylamine maleate | ChEBI |
| (+)-Brompheniramine maleate | ChEBI |
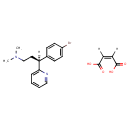
| (3S)-3-(4-Bromophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine maleate | ChEBI |
| (S)-1-(p-Bromophenyl)-1-(2-pyridyl)-3-dimethylaminopropane maleate | ChEBI |
| (S)-2-(p-Bromo-alpha-(2-dimethylaminoethyl)benzyl)pyridine maleate | ChEBI |
| (S)-3-(4-Bromophenyl)-N,N-dimethyl-3-(2-pyridinyl)-1-propanamine maleate | ChEBI |
| (S)-3-(4-Bromophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine maleate | ChEBI |
| (S)-3-(p-Bromophenyl)-3-(2-pyridyl)-N,N-dimethylpropylamine maleate | ChEBI |
| Disomer | Kegg |
| (+)-2-(p-Bromo-a-(2-dimethylaminoethyl)benzyl)pyridine maleate | Generator |
| (+)-2-(p-Bromo-a-(2-dimethylaminoethyl)benzyl)pyridine maleic acid | Generator |
| (+)-2-(p-Bromo-alpha-(2-dimethylaminoethyl)benzyl)pyridine maleic acid | Generator |
| (+)-2-(p-Bromo-α-(2-dimethylaminoethyl)benzyl)pyridine maleate | Generator |
| (+)-2-(p-Bromo-α-(2-dimethylaminoethyl)benzyl)pyridine maleic acid | Generator |
| (+)-3-(4-Bromophenyl)-N,N-dimethyl-3-(2-pyridinyl)-1-propanamine maleic acid | Generator |
| (+)-3-(4-Bromophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine maleic acid | Generator |
| (+)-3-(p-Bromophenyl)-3-(2-pyridyl)-N,N-dimethylpropylamine maleic acid | Generator |
| (+)-Brompheniramine maleic acid | Generator |
| (3S)-3-(4-Bromophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine maleic acid | Generator |
| (S)-1-(p-Bromophenyl)-1-(2-pyridyl)-3-dimethylaminopropane maleic acid | Generator |
| (S)-2-(p-Bromo-a-(2-dimethylaminoethyl)benzyl)pyridine maleate | Generator |
| (S)-2-(p-Bromo-a-(2-dimethylaminoethyl)benzyl)pyridine maleic acid | Generator |
| (S)-2-(p-Bromo-alpha-(2-dimethylaminoethyl)benzyl)pyridine maleic acid | Generator |
| (S)-2-(p-Bromo-α-(2-dimethylaminoethyl)benzyl)pyridine maleate | Generator |
| (S)-2-(p-Bromo-α-(2-dimethylaminoethyl)benzyl)pyridine maleic acid | Generator |
| (S)-3-(4-Bromophenyl)-N,N-dimethyl-3-(2-pyridinyl)-1-propanamine maleic acid | Generator |
| (S)-3-(4-Bromophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine maleic acid | Generator |
| (S)-3-(p-Bromophenyl)-3-(2-pyridyl)-N,N-dimethylpropylamine maleic acid | Generator |
| Dexbrompheniramine maleic acid | Generator |
| (3S)-3-(4-Bromophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine;(Z)-but-2-enedioate | Generator |
| Disophrol | MeSH |
| Dexbrompheniramine | MeSH |
| Dexbrompheniramine maleate | MeSH |