| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:20:45 UTC |
|---|
| Update Date | 2016-11-09 01:16:02 UTC |
|---|
| Accession Number | CHEM019623 |
|---|
| Identification |
|---|
| Common Name | Benzathine penicillin G |
|---|
| Class | Small Molecule |
|---|
| Description | A benzathine(2+) salt in which the counter anions are benzylpenicillin(1-). Drug-of-choice when prolonged low concentrations of benzylpenicillin are required and appropriate. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
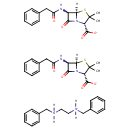
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Beacillin | ChEBI | | Benzathine benzylpenicillin | ChEBI | | Benzathine benzylpenicilline | ChEBI | | Benzathini benzylpenicillinum | ChEBI | | Benzatina bencilpenicilina | ChEBI | | Benzylpenicillin dibenzylethylenediamine salt | ChEBI | | Bicillin | ChEBI | | Bicillin L-a | ChEBI | | Cepacilina | ChEBI | | Extencilline | ChEBI | | Lentopenil | ChEBI | | N,N'-dibenzylethylenediamine bis(benzyl penicillin) | ChEBI | | Penicillin g benzathine | ChEBI | | Penicillin g benzathine anhydrous | ChEBI | | Penicillin g salt OF N,n'-dibenzylethylenediamine | ChEBI | | Penidural | ChEBI | | Permapen | ChEBI | | Tardocillin | ChEBI | | Benzathine benzylpénicilline panpharma | MeSH | | Benzetacil | MeSH | | Penduran | MeSH | | Bicillin forte | MeSH | | Benzathine benzylpenicillin, procaine benzylpenicillin, benzylpenicillin sodium drug combination | MeSH | | Benzathine benzylpenicillin, procaine benzylpenicillin drug combination | MeSH | | Bicillin-3 | MeSH | | Benzathine benzylpenicillin, procaine benzylpenicillin, drug combination | MeSH | | Bencelin | MeSH | | Bicillin la | MeSH | | Benzathine benzylpenicillin - procaine benzylpenicillin | MeSH | | Bicillin III | MeSH | | Benzathine benzylpenicillin, procaine benzylpenicillin, benzylpenicillin potassium drug combination | MeSH | | Bicillin C-R | MeSH | | Debecillin | MeSH | | Peniroger retard | MeSH | | Brevicilina | MeSH | | Bicillin 1 | MeSH | | Benzathine, penicillin g | MeSH | | Provipen benzatina | MeSH | | Benzylpenicillin, benzathine | MeSH | | Retacillin | MeSH | | Bicillin L a | MeSH | | Pendysin | MeSH | | Benzathine penicillin | MeSH | | Pendepon | MeSH | | Retazillin | MeSH | | Penicillin, benzathine | MeSH | | N,N'-dibenzylethane-1,2-diamine;(2S,5R,6R)-3,3-dimethyl-7-oxo-6-[(2-phenylacetyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate | Generator | | Antibioticos brand OF benzathine benzylpenicillin | MeSH | | CSL Brand OF benzathine benzylpenicillin | MeSH | | g Benzathine, penicillin | MeSH | | I farmacologia brand OF benzathine benzylpenicillin | MeSH | | Roger brand OF benzathine benzylpenicillin | MeSH | | Sandoz brand OF penicillin g benzathine | MeSH | | Antibioticos farma brand OF penicillin g benzathine | MeSH | | King pharmaceuticals brand OF penicillin g benzathine | MeSH | | LPG | MeSH | | Roerig brand OF benzathine benzylpenicillin | MeSH | | Jenapharm brand OF benzathine benzylpenicillin | MeSH | | Wyeth brand OF penicillin g benzathine | MeSH | | Bayer brand OF benzathine benzylpenicillin | MeSH | | Panpharma brand OF benzathine benzylpenicillin | MeSH | | Sabater brand OF benzathine benzylpenicillin | MeSH | | Specia brand OF benzathine benzylpenicillin | MeSH | | Wassermann brand OF benzathine benzylpenicillin | MeSH | | Yamanouchi brand OF benzathine benzylpenicillin | MeSH |
|
|---|
| Chemical Formula | C48H56N6O8S2 |
|---|
| Average Molecular Mass | 909.130 g/mol |
|---|
| Monoisotopic Mass | 908.360 g/mol |
|---|
| CAS Registry Number | 1538-09-6 |
|---|
| IUPAC Name | benzyl[2-(benzylazaniumyl)ethyl]azanium bis((2S,5R,6R)-3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate) |
|---|
| Traditional Name | benzathine(2+); bis(benzylpenicillin(1-)) |
|---|
| SMILES | [H][N+]([H])(CC[N+]([H])([H])CC1=CC=CC=C1)CC1=CC=CC=C1.[H][C@]12SC(C)(C)[C@@H](N1C(=O)[C@H]2NC(=O)CC1=CC=CC=C1)C([O-])=O.[H][C@]12SC(C)(C)[C@@H](N1C(=O)[C@H]2NC(=O)CC1=CC=CC=C1)C([O-])=O |
|---|
| InChI Identifier | InChI=1S/2C16H18N2O4S.C16H20N2/c2*1-16(2)12(15(21)22)18-13(20)11(14(18)23-16)17-10(19)8-9-6-4-3-5-7-9;1-3-7-15(8-4-1)13-17-11-12-18-14-16-9-5-2-6-10-16/h2*3-7,11-12,14H,8H2,1-2H3,(H,17,19)(H,21,22);1-10,17-18H,11-14H2/t2*11-,12+,14-;/m11./s1 |
|---|
| InChI Key | BVGLIYRKPOITBQ-ANPZCEIESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 4-aminoquinolines. These are organic compounds containing an amino group attached to the 4-position of a quinoline ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Aminoquinolines and derivatives |
|---|
| Direct Parent | 4-aminoquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4-aminoquinoline
- Haloquinoline
- Chloroquinoline
- Aminopyridine
- Sulfuric acid
- Secondary aliphatic/aromatic amine
- Aryl chloride
- Aryl halide
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Organic sulfuric acid or derivatives
- Tertiary aliphatic amine
- Tertiary amine
- 1,2-aminoalcohol
- Azacycle
- Secondary amine
- Alkanolamine
- Organonitrogen compound
- Organochloride
- Organopnictogen compound
- Organic oxygen compound
- Organohalogen compound
- Alcohol
- Amine
- Organic nitrogen compound
- Organic oxide
- Organooxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000000009-d9e6081c3e23c83a3d9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000000009-d9e6081c3e23c83a3d9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0000000009-d9e6081c3e23c83a3d9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000000009-473ba73c7846e1685b75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000000009-473ba73c7846e1685b75 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0000000009-473ba73c7846e1685b75 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB09323 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Benzathine_benzylpenicillin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 51352 |
|---|
| PubChem Compound ID | 15232 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|