| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:04:33 UTC |
|---|
| Update Date | 2016-11-09 01:15:59 UTC |
|---|
| Accession Number | CHEM019396 |
|---|
| Identification |
|---|
| Common Name | 2-sec-Butyl-3-methoxypyrazine |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Methoxy-3-(1-methylpropyl)pyrazine is found in alcoholic beverages. 2-Methoxy-3-(1-methylpropyl)pyrazine is a volatile component of many vegetables, e.g. asparagus, carrot, celery, cucumber, parsnip, bell peppers and pea, also in ginger, galbanum oil and white wine. 2-Methoxy-3-(1-methylpropyl)pyrazine is a flavouring ingredient. |
|---|
| Contaminant Sources | - FooDB Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
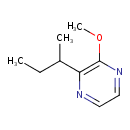
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Methoxy-3-(1-methyl-propyl) pyrazine | HMDB | | 2-Methoxy-3-(1-methylpropyl)-pyrazine | HMDB | | 2-Methoxy-3-sec-butylpyrazine | HMDB | | 2-Sec-butyl-3-methoxy-pyrazine | HMDB | | 2-Sec-butyl-3-methoxypyrazine | HMDB | | 2-Sec-butyl-3-methoxypyrazine, 8ci | HMDB | | 3-Sec-butyl-2-methoxypyrazine | HMDB | | FEMA 3433 | HMDB | | Pyrazine, 2-methoxy, 3-sec-butyl | HMDB | | Pyrazine, 3-methoxy-2-(1-methylpropyl) | HMDB |
|
|---|
| Chemical Formula | C9H14N2O |
|---|
| Average Molecular Mass | 166.220 g/mol |
|---|
| Monoisotopic Mass | 166.111 g/mol |
|---|
| CAS Registry Number | 24168-70-5 |
|---|
| IUPAC Name | 2-(butan-2-yl)-3-methoxypyrazine |
|---|
| Traditional Name | 2-methoxy-3-(sec-butyl)pyrazine |
|---|
| SMILES | CCC(C)C1=C(OC)N=CC=N1 |
|---|
| InChI Identifier | InChI=1S/C9H14N2O/c1-4-7(2)8-9(12-3)11-6-5-10-8/h5-7H,4H2,1-3H3 |
|---|
| InChI Key | QMQDJVIJVPEQHE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methoxypyrazines. These are pyrazines containing a methoxyl group attached to the pyrazine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrazines |
|---|
| Direct Parent | Methoxypyrazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methoxypyrazine
- Alkyl aryl ether
- Heteroaromatic compound
- Azacycle
- Ether
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ug3-8900000000-d59c6a45a3f59420ccff | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-588fd221c9b82f2ca254 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-eb2eb7b0011b666a98f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-47ae7eed3f45f15872b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1900000000-12c585e05f3b97e2a828 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-5900000000-4826268dbad43e1b337e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-9100000000-70f488276a3d77293eb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-1ec7efe000cc5c76adc6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-4900000000-490ccca717f62200a06e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-9200000000-63fe40d2a764a4987302 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-c06e07ad75c433b516d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-1900000000-5c938f56dd8a4c73cd79 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9200000000-7a447dbfe785f322c052 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0079-4900000000-a56732b45eb5203cad2c | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0032933 |
|---|
| FooDB ID | FDB010918 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 453664 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 520098 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB01782 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|