| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:02:25 UTC |
|---|
| Update Date | 2016-11-09 01:15:58 UTC |
|---|
| Accession Number | CHEM019346 |
|---|
| Identification |
|---|
| Common Name | Azosemide |
|---|
| Class | Small Molecule |
|---|
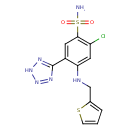
| Description | A sulfonamide that is benzenesulfonamide which is substituted at positions 2, 4, and 5 by chlorine, (2-thienylmethyl)amino and 1H-tetrazol-5-yl groups, respectively. It is a diuretic that has been used in the management of oedema and hypertension. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Chloro-5-(1H-tetrazol-5-yl)-N(4)-2-thenylsulfanilamide | ChEBI | | 5-(4'-Chloro-5'-sulfamoyl-2'-thenylaminophenyl)tetrazole | ChEBI | | Azosemida | ChEBI | | Azosemidum | ChEBI | | Diart | ChEBI | | 2-Chloro-5-(1H-tetrazol-5-yl)-N(4)-2-thenylsulphanilamide | Generator | | 5-(4'-Chloro-5'-sulphamoyl-2'-thenylaminophenyl)tetrazole | Generator | | 2-Chloro-5-(1H-tetrazol-5-yl)-4-[(thiophen-2-ylmethyl)amino]benzenesulfonamide | HMDB | | 2-Chloro-5-(1H-tetrazol-5-yl)-N4-2-thenylsulfanilamide | HMDB | | Azosemid | HMDB | | 5-(4-Chloro-5-sulfamyl-2-thienylaminophenyl)tetrazole | HMDB |
|
|---|
| Chemical Formula | C12H11ClN6O2S2 |
|---|
| Average Molecular Mass | 370.838 g/mol |
|---|
| Monoisotopic Mass | 370.007 g/mol |
|---|
| CAS Registry Number | 27589-33-9 |
|---|
| IUPAC Name | 2-chloro-5-(2H-1,2,3,4-tetrazol-5-yl)-4-[(thiophen-2-ylmethyl)amino]benzene-1-sulfonamide |
|---|
| Traditional Name | azosemide |
|---|
| SMILES | NS(=O)(=O)C1=C(Cl)C=C(NCC2=CC=CS2)C(=C1)C1=NNN=N1 |
|---|
| InChI Identifier | InChI=1S/C12H11ClN6O2S2/c13-9-5-10(15-6-7-2-1-3-22-7)8(12-16-18-19-17-12)4-11(9)23(14,20)21/h1-5,15H,6H2,(H2,14,20,21)(H,16,17,18,19) |
|---|
| InChI Key | HMEDEBAJARCKCT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenyltetrazoles and derivatives. Phenyltetrazoles and derivatives are compounds containing a phenyltetrazole skeleton, which consists of a tetrazole bound to a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Tetrazoles |
|---|
| Direct Parent | Phenyltetrazoles and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminobenzenesulfonamide
- Phenyltetrazole
- Benzenesulfonamide
- Benzenesulfonyl group
- Aniline or substituted anilines
- Phenylalkylamine
- Chlorobenzene
- Halobenzene
- Secondary aliphatic/aromatic amine
- Aralkylamine
- Aryl chloride
- Benzenoid
- Organosulfonic acid amide
- Aryl halide
- Monocyclic benzene moiety
- Aminosulfonyl compound
- Thiophene
- Sulfonyl
- Organosulfonic acid or derivatives
- Organic sulfonic acid or derivatives
- Heteroaromatic compound
- Secondary amine
- Azacycle
- Organopnictogen compound
- Organochloride
- Organohalogen compound
- Organic nitrogen compound
- Amine
- Organic oxygen compound
- Organonitrogen compound
- Organosulfur compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-3179000000-ab9cce02c68a2faedfa5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0019000000-4235353754d07f2eabb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00g0-0059000000-3942e6570eaabb2100d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-054k-0191000000-9f7de4073d6c720917a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-2009000000-49efb527af3e3895d8c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00or-9015000000-2e972e4ca5c8921631f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-f2169c50df45a7b901e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-78304d7d04dcd1a36029 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00pr-6019000000-8c32e4c4e0ad74156f00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9011000000-5a32e360207da8b1e194 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-d48728f26a4fcf28a99d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0009000000-3c3da5c3b9c593642134 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ls-6089000000-b92cde7661137efad26e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB08961 |
|---|
| HMDB ID | HMDB0041831 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Azosemide |
|---|
| Chemspider ID | 2186 |
|---|
| ChEBI ID | 31248 |
|---|
| PubChem Compound ID | 2273 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|