| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:01:39 UTC |
|---|
| Update Date | 2016-11-09 01:15:58 UTC |
|---|
| Accession Number | CHEM019326 |
|---|
| Identification |
|---|
| Common Name | Trabectedin |
|---|
| Class | Small Molecule |
|---|
| Description | Trabectedin, also referred as Trabectedin during its development, is a marine-derived antitumor agent discovered in the Carribean tunicate _Ecteinascidia turbinata_ and now produced synthetically. Trabectedin has a unique mechanism of action. It binds to the minor groove of DNA interfering with cell division and genetic transcription processes and DNA repair machinery. It is approved for use in Europe, Russia and South Korea for the treatment of advanced soft tissue sarcoma. It is currently under evaluation for the treatment of breast cancer, prostate cancer, in addition to pediatric sarcomas. Both the European Commission and the U.S. Food and Drug Administration (FDA) have approved trabectedin as an orphan drug in soft tissue sarcomas and ovarian cancer. On October 23, 2015, the FDA approved trabectedin, (as Yondelis), for the treatment of specific soft tissue sarcomas. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
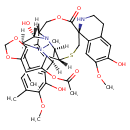
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ect 743 | ChEBI | | Ecteinascidin | ChEBI | | Ecteinascidin 743 | ChEBI | | ET-743 | ChEBI | | ET743 | ChEBI | | Yondelis | ChEBI | | NSC 684766 | HMDB |
|
|---|
| Chemical Formula | C39H43N3O11S |
|---|
| Average Molecular Mass | 761.837 g/mol |
|---|
| Monoisotopic Mass | 761.262 g/mol |
|---|
| CAS Registry Number | 114899-77-3 |
|---|
| IUPAC Name | (1R,2R,3R,11S,12S,14R,26R)-5,6',12-trihydroxy-6,7'-dimethoxy-7,21,30-trimethyl-27-oxo-3',4'-dihydro-2'H-17,19,28-trioxa-24-thia-13,30-diazaspiro[heptacyclo[12.9.6.1³,¹¹.0²,¹³.0⁴,⁹.0¹⁵,²³.0¹⁶,²⁰]triacontane-26,1'-isoquinoline]-4,6,8,15,20,22-hexaen-22-yl acetate |
|---|
| Traditional Name | yondelis |
|---|
| SMILES | [H][C@@]12[C@@H]3SC[C@]4(NCCC5=C4C=C(OC)C(O)=C5)C(=O)OC[C@H](N1[C@@H](O)[C@@H]1CC4=CC(C)=C(OC)C(O)=C4[C@H]2N1C)C1=C2OCOC2=C(C)C(OC(C)=O)=C31 |
|---|
| InChI Identifier | InChI=1S/C39H43N3O11S/c1-16-9-20-10-22-37(46)42-23-13-50-38(47)39(21-12-25(48-5)24(44)11-19(21)7-8-40-39)14-54-36(30(42)29(41(22)4)26(20)31(45)32(16)49-6)28-27(23)35-34(51-15-52-35)17(2)33(28)53-18(3)43/h9,11-12,22-23,29-30,36-37,40,44-46H,7-8,10,13-15H2,1-6H3/t22-,23-,29+,30+,36+,37-,39+/m0/s1 |
|---|
| InChI Key | PKVRCIRHQMSYJX-AIFWHQITSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzazocines. These are organic compounds containing the benzazocine ring system, which consists of a benzene ring bound to an azocine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzazocines |
|---|
| Direct Parent | Benzazocines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzazocine
- Tetrahydroisoquinoline
- Alpha-amino acid or derivatives
- Benzodioxole
- Anisole
- Alkyl aryl ether
- N-methylpiperazine
- N-alkylpiperazine
- Aralkylamine
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Dicarboxylic acid or derivatives
- Piperazine
- 1,4-diazinane
- Hemiaminal
- Lactone
- Tertiary aliphatic amine
- Carboxylic acid ester
- Tertiary amine
- Amino acid or derivatives
- Acetal
- Alkanolamine
- Carboxylic acid derivative
- Oxacycle
- Secondary aliphatic amine
- Azacycle
- Organoheterocyclic compound
- Ether
- Dialkylthioether
- Thioether
- Polyol
- Secondary amine
- Amine
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0zgr-0090000100-1fbe6c879e491f9c397c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000000900-e91cca9b4689a68d194c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0300002900-083c4ab0de6a8cbf080d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-2130029700-488b00bc8602ecbb0612 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000000900-5dd80d9620ee90346daa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-11ou-1000001900-ea86efb65818a8f27be5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kac-6050009800-1902e4760cf83e432109 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000000900-cf19b0cf6f95b02564a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000000900-3e1fdba9b1bd766aa3dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00vl-0010008900-6487d80738403778f792 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000000900-2edcd395ca8015613eae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0000000900-2874db466c2b035d495b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01tl-0200016900-85e72ee9c89dddad28e0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB05109 |
|---|
| HMDB ID | HMDB0015609 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | ECT |
|---|
| Wikipedia Link | Trabectedin |
|---|
| Chemspider ID | 97236 |
|---|
| ChEBI ID | 84050 |
|---|
| PubChem Compound ID | 108150 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Authors unspecified: Trabectedin: Ecteinascidin 743, Ecteinascidin-743, ET 743, ET-743, NSC 684766. Drugs R D. 2006;7(5):317-28. | | 2. Carter NJ, Keam SJ: Trabectedin : a review of its use in the management of soft tissue sarcoma and ovarian cancer. Drugs. 2007;67(15):2257-76. | | 3. Krasner CN, McMeekin DS, Chan S, Braly PS, Renshaw FG, Kaye S, Provencher DM, Campos S, Gore ME: A Phase II study of trabectedin single agent in patients with recurrent ovarian cancer previously treated with platinum-based regimens. Br J Cancer. 2007 Dec 17;97(12):1618-24. Epub 2007 Nov 13. | | 4. Tavecchio M, Natoli C, Ubezio P, Erba E, D'Incalci M: Dynamics of cell cycle phase perturbations by trabectedin (ET-743) in nucleotide excision repair (NER)-deficient and NER-proficient cells, unravelled by a novel mathematical simulation approach. Cell Prolif. 2007 Dec;40(6):885-904. | | 5. Zhou SF, Zhou ZW, Yang LP, Cai JP: Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. Epub 2009 Sep 1. | | 6. Guirouilh-Barbat J, Antony S, Pommier Y: Zalypsis (PM00104) is a potent inducer of gamma-H2AX foci and reveals the importance of the C ring of trabectedin for transcription-coupled repair inhibition. Mol Cancer Ther. 2009 Jul;8(7):2007-14. doi: 10.1158/1535-7163.MCT-09-0336. Epub 2009 Jul 7. | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=21403840 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=21499557 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=23149213 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=24001124 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=24277455 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=24662672 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=24692579 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=24696229 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=24707261 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=24755886 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=24756367 | | 18. https://www.ncbi.nlm.nih.gov/pubmed/?term=24803896 | | 19. https://www.ncbi.nlm.nih.gov/pubmed/?term=24844217 | | 20. https://www.ncbi.nlm.nih.gov/pubmed/?term=24917554 | | 21. https://www.ncbi.nlm.nih.gov/pubmed/?term=24941346 | | 22. https://www.ncbi.nlm.nih.gov/pubmed/?term=24982387 | | 23. https://www.ncbi.nlm.nih.gov/pubmed/?term=25048043 | | 24. https://www.ncbi.nlm.nih.gov/pubmed/?term=25048044 | | 25. https://www.ncbi.nlm.nih.gov/pubmed/?term=25050069 | | 26. https://www.ncbi.nlm.nih.gov/pubmed/?term=25076252 | | 27. https://www.ncbi.nlm.nih.gov/pubmed/?term=25100135 | | 28. https://www.ncbi.nlm.nih.gov/pubmed/?term=25209722 | | 29. https://www.ncbi.nlm.nih.gov/pubmed/?term=25263179 | | 30. https://www.ncbi.nlm.nih.gov/pubmed/?term=25273374 | | 31. https://www.ncbi.nlm.nih.gov/pubmed/?term=25479910 |
|
|---|