| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 06:00:02 UTC |
|---|
| Update Date | 2016-11-09 01:15:58 UTC |
|---|
| Accession Number | CHEM019304 |
|---|
| Identification |
|---|
| Common Name | Tamibarotene |
|---|
| Class | Small Molecule |
|---|
| Description | Tamibarotene is a novel synthetic retinoid for acute promyelocytic leukaemia (APL). Tamibarotene is currently approved in Japan for treatment of recurrent APL, and is undergoing clinical trials in the United States. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
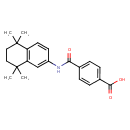
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Am 80 | ChEBI | | Am-80 | ChEBI | | Amnoid | ChEBI | | Retinobenzoic acid | ChEBI | | Tamibaro | ChEBI | | Retinobenzoate | Generator | | 4-((5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)carbamoyl)benzoic acid | HMDB |
|
|---|
| Chemical Formula | C22H25NO3 |
|---|
| Average Molecular Mass | 351.439 g/mol |
|---|
| Monoisotopic Mass | 351.183 g/mol |
|---|
| CAS Registry Number | 94497-51-5 |
|---|
| IUPAC Name | 4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)carbamoyl]benzoic acid |
|---|
| Traditional Name | tamibarotene |
|---|
| SMILES | CC1(C)CCC(C)(C)C2=C1C=CC(NC(=O)C1=CC=C(C=C1)C(O)=O)=C2 |
|---|
| InChI Identifier | InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) |
|---|
| InChI Key | MUTNCGKQJGXKEM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetralins. These are polycyclic aromatic compounds containing a tetralin moiety, which consists of a benzene fused to a cyclohexane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Tetralins |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetralins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetralin
- Benzamide
- Benzoic acid or derivatives
- Benzoic acid
- Benzoyl
- Monocyclic benzene moiety
- Secondary carboxylic acid amide
- Carboxamide group
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-0926000000-4e20d564460f24f3bee6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-3195400000-2f9ea0b192db22b23e34 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-8077c73aa7a0b3a0493f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pb9-1119000000-0596ca0316feae6135e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00bc-9621000000-472a8ced02b76b7e0310 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-3ef35313b76eb3af9c7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0009000000-986ece158863bf5eb6b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-2090000000-faa1fb3b6d57e52a9c4c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0009000000-dbfed4dced06a8666457 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053r-1239000000-eb277707477e13926eb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kii-4930000000-87f3d164d219001d818b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0019000000-d9e09ce3c5ade8ebd024 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gwb-0369000000-6c232cbaf08e18c9b277 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-3921000000-fbd8a12bd61c31548a10 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04942 |
|---|
| HMDB ID | HMDB0015605 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | A80 |
|---|
| Wikipedia Link | Tamibarotene |
|---|
| Chemspider ID | 97231 |
|---|
| ChEBI ID | 32181 |
|---|
| PubChem Compound ID | 108143 |
|---|
| Kegg Compound ID | C12864 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Mizojiri K, Okabe H, Sugeno K, Misaki A, Ito M, Kominami G, Esumi Y, Takaichi M, Harada T, Seki H, Inaba A: Studies on the metabolism and disposition of the new retinoid 4-[(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthyl)carbamoyl] benzoic acid. 4th communication: absorption, metabolism, excretion and plasma protein binding in various animals and man. Arzneimittelforschung. 1997 Mar;47(3):259-69. | | 2. Authors unspecified: Tamibarotene: AM 80, retinobenzoic acid, Tamibaro. Drugs R D. 2004;5(6):359-62. | | 3. Sanda T, Kuwano T, Nakao S, Iida S, Ishida T, Komatsu H, Shudo K, Kuwano M, Ono M, Ueda R: Antimyeloma effects of a novel synthetic retinoid Am80 (Tamibarotene) through inhibition of angiogenesis. Leukemia. 2005 Jun;19(6):901-9. | | 4. Takeuchi M: [Clinical experience with a new synthetic retinoid, tamibarotene (Am-80) for relapsed or refractory acute promyelocytic leukemia]. Gan To Kagaku Ryoho. 2006 Mar;33(3):397-401. | | 5. Miwako I, Kagechika H: Tamibarotene. Drugs Today (Barc). 2007 Aug;43(8):563-8. | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=16531727 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=17925887 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=19182380 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=19389933 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=19514122 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=22014829 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=22863914 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=23404745 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=25448496 |
|
|---|