| Synonyms | | Value | Source |
|---|
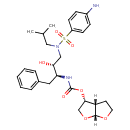
| (3R,3AS,6ar)-hexahydrofuro[2,3-b]furan-3-yl N-((1S,2R)-1-benzyl-2-hydroxy-3-(N(1)-isobutylsulfanilamido)propyl)carbamate | ChEBI | | (3R,3AS,6ar)-hexahydrofuro[2,3-b]furan-3-yl(1S,2R)-3-[[(4-aminophenyl)sulfonyl](isobutyl)amino]-1-benzyl-2-hydroxypropylcarbamATE | ChEBI | | (3R,3AS,6ar)-tetrahydro-2H-furo[2,3-b]furan-3-yl (2S,3R)-4-(4-amino-N-isobutylphenylsulfonamido)-3-hydroxy-1-phenylbutan-2-ylcarbamate | ChEBI | | (3R,3AS,6ar)-tetrahydro-2H-furo[2,3-b]furan-3-yl (2S,3R)-4-(4-amino-N-neopentylphenylsulfonamido)-3-hydroxy-1-phenylbutan-2-ylcarbamate | ChEBI | | [(S)-3-[(4-Amino-benzenesulfonyl)-isobutyl-amino]-2-hydroxy-1-((R)-phenylmethyl)-propyl]-carbamic acid (3R,3as,6ar)-(hexahydro-furo[2,3-b]furan-3-yl) ester | ChEBI | | Darunavirum | ChEBI | | N-((1S,2R)-3-(((4-Aminophenyl)sulfonyl)(2-methylpropyl)amino)-2-hydroxy-1-benzylpropyl)((1S,2R,5R)-4,6-dioxabicyclo(3.3.0)oct-2-yloxy)carboxamide | ChEBI | | TMC114 | ChEBI | | {(1S,2R)-3-[(4-amino-benzenesulfonyl)-isobutyl-amino]-1-benzyl-2-hydroxy-propyl}-carbamic acid (3R,3as,6ar)-(hexahydro-furo[2,3-b]furan-3-yl) ester | ChEBI | | (3R,3AS,6ar)-hexahydrofuro[2,3-b]furan-3-yl N-((1S,2R)-1-benzyl-2-hydroxy-3-(N(1)-isobutylsulfanilamido)propyl)carbamic acid | Generator | | (3R,3AS,6ar)-hexahydrofuro[2,3-b]furan-3-yl N-((1S,2R)-1-benzyl-2-hydroxy-3-(N(1)-isobutylsulphanilamido)propyl)carbamate | Generator | | (3R,3AS,6ar)-hexahydrofuro[2,3-b]furan-3-yl N-((1S,2R)-1-benzyl-2-hydroxy-3-(N(1)-isobutylsulphanilamido)propyl)carbamic acid | Generator | | (3R,3AS,6ar)-hexahydrofuro[2,3-b]furan-3-yl(1S,2R)-3-[[(4-aminophenyl)sulfonyl](isobutyl)amino]-1-benzyl-2-hydroxypropylcarbamic acid | Generator | | (3R,3AS,6ar)-hexahydrofuro[2,3-b]furan-3-yl(1S,2R)-3-[[(4-aminophenyl)sulphonyl](isobutyl)amino]-1-benzyl-2-hydroxypropylcarbamate | Generator | | (3R,3AS,6ar)-hexahydrofuro[2,3-b]furan-3-yl(1S,2R)-3-[[(4-aminophenyl)sulphonyl](isobutyl)amino]-1-benzyl-2-hydroxypropylcarbamic acid | Generator | | (3R,3AS,6ar)-tetrahydro-2H-furo[2,3-b]furan-3-yl (2S,3R)-4-(4-amino-N-isobutylphenylsulfonamido)-3-hydroxy-1-phenylbutan-2-ylcarbamic acid | Generator | | (3R,3AS,6ar)-tetrahydro-2H-furo[2,3-b]furan-3-yl (2S,3R)-4-(4-amino-N-isobutylphenylsulphonamido)-3-hydroxy-1-phenylbutan-2-ylcarbamate | Generator | | (3R,3AS,6ar)-tetrahydro-2H-furo[2,3-b]furan-3-yl (2S,3R)-4-(4-amino-N-isobutylphenylsulphonamido)-3-hydroxy-1-phenylbutan-2-ylcarbamic acid | Generator | | (3R,3AS,6ar)-tetrahydro-2H-furo[2,3-b]furan-3-yl (2S,3R)-4-(4-amino-N-neopentylphenylsulfonamido)-3-hydroxy-1-phenylbutan-2-ylcarbamic acid | Generator | | (3R,3AS,6ar)-tetrahydro-2H-furo[2,3-b]furan-3-yl (2S,3R)-4-(4-amino-N-neopentylphenylsulphonamido)-3-hydroxy-1-phenylbutan-2-ylcarbamate | Generator | | (3R,3AS,6ar)-tetrahydro-2H-furo[2,3-b]furan-3-yl (2S,3R)-4-(4-amino-N-neopentylphenylsulphonamido)-3-hydroxy-1-phenylbutan-2-ylcarbamic acid | Generator | | [(S)-3-[(4-Amino-benzenesulfonyl)-isobutyl-amino]-2-hydroxy-1-((R)-phenylmethyl)-propyl]-carbamate (3R,3as,6ar)-(hexahydro-furo[2,3-b]furan-3-yl) ester | Generator | | [(S)-3-[(4-Amino-benzenesulphonyl)-isobutyl-amino]-2-hydroxy-1-((R)-phenylmethyl)-propyl]-carbamate (3R,3as,6ar)-(hexahydro-furo[2,3-b]furan-3-yl) ester | Generator | | [(S)-3-[(4-Amino-benzenesulphonyl)-isobutyl-amino]-2-hydroxy-1-((R)-phenylmethyl)-propyl]-carbamic acid (3R,3as,6ar)-(hexahydro-furo[2,3-b]furan-3-yl) ester | Generator | | N-((1S,2R)-3-(((4-Aminophenyl)sulphonyl)(2-methylpropyl)amino)-2-hydroxy-1-benzylpropyl)((1S,2R,5R)-4,6-dioxabicyclo(3.3.0)oct-2-yloxy)carboxamide | Generator | | {(1S,2R)-3-[(4-amino-benzenesulfonyl)-isobutyl-amino]-1-benzyl-2-hydroxy-propyl}-carbamate (3R,3as,6ar)-(hexahydro-furo[2,3-b]furan-3-yl) ester | Generator | | {(1S,2R)-3-[(4-amino-benzenesulphonyl)-isobutyl-amino]-1-benzyl-2-hydroxy-propyl}-carbamate (3R,3as,6ar)-(hexahydro-furo[2,3-b]furan-3-yl) ester | Generator | | {(1S,2R)-3-[(4-amino-benzenesulphonyl)-isobutyl-amino]-1-benzyl-2-hydroxy-propyl}-carbamic acid (3R,3as,6ar)-(hexahydro-furo[2,3-b]furan-3-yl) ester | Generator | | AIDS073035 | HMDB | | TMC-114 | HMDB | | UIC-94017 | HMDB | | 114, TMC | HMDB | | TMC 114 | HMDB | | Ethanolate, darunavir | HMDB | | Darunavir ethanolate | HMDB | | Prezista | HMDB |
|
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-7395120000-9ee6643d8e957bfc6883 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-08i3-8659131000-a5895dfe8fa0c5374f22 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Darunavir,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0aor-0900800000-e427a102e3a7551cc732 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0a4i-0900000000-8ab8c2b3421f4a277282 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0a4i-2900000000-eddacc917eaf16abe9d2 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0a4i-2900000000-86c785caeafe3b4a10c3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0a4l-5900000000-69bc90a59383cb5a804b | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0a4l-8900000000-f481d0932a99692c87d9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0a4i-0900000000-40373637b670c5395ffd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-0aor-0900800000-f59913cdbcd62042530a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-0a4i-2900000000-10dadadeebe1e6ec0164 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-000g-0009760000-f730e27547a482694ebc | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0006-0009000000-fcd18a6f4e9084f0f8f1 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0006-0469000000-716a2ae87ad9df6765fb | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0a4i-0941000000-5e0dbe51025009642b33 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0a4i-0910000000-2411514d55bfa3c4e81f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0006-2309000000-e6767f5d8648334c41c9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-014i-9621000000-3a7c794f5e3f473b9ad5 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-014i-9400000000-16b363cdd836532460ce | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0a4i-2900000000-98bfa9c688e4cb2dbbc3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0a4l-4900000000-05c5a10ff111a5d1c9bf | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-052f-6900000000-f59c1c0d97f0a8ab00d7 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0009000000-8e1cc4b8ca6f49bcfe56 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-000g-0009760000-26cdc56a39511d44e965 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-0006-2309000000-b56fe3acae7c21ddaba0 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0941000000-5e0dbe51025009642b33 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0006-0469000000-c470e3e505d43b7089af | Spectrum |
|
|---|