| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:56:12 UTC |
|---|
| Update Date | 2016-11-09 01:15:57 UTC |
|---|
| Accession Number | CHEM019233 |
|---|
| Identification |
|---|
| Common Name | Ethylmorphine |
|---|
| Class | Small Molecule |
|---|
| Description | A narcotic analgesic and antitussive. It is metabolized in the liver by ethylmorphine-N-demethylase and used as an indicator of liver function. It is not marketed in the US but is approved for use in various countries around the world. In the US it is a schedule II drug (single-entity) and schedule III drug (in combination products). |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
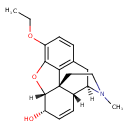
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dionine | HMDB | | Ethyl morphine | HMDB | | Ethylmorphine, (5alpha,6beta)-isomer | HMDB | | Novartis brand OF ethylmorphine hydrochloride, (5alpha,6alpha)-isomer | HMDB | | Trachyl | HMDB | | Ethomorphine | HMDB | | Ethylmorphine hydrochloride, (5alpha,6alpha)-isomer | HMDB | | Ethylmorphine sulfate (2:1), (5alpha,6alpha)-isomer | HMDB | | Ethylmorphine hydrochloride, dihydrate, (5alpha,6alpha)-isomer | HMDB |
|
|---|
| Chemical Formula | C19H23NO3 |
|---|
| Average Molecular Mass | 313.391 g/mol |
|---|
| Monoisotopic Mass | 313.168 g/mol |
|---|
| CAS Registry Number | 76-58-4 |
|---|
| IUPAC Name | (1S,5R,13R,14S,17R)-10-ethoxy-4-methyl-12-oxa-4-azapentacyclo[9.6.1.0¹,¹³.0⁵,¹⁷.0⁷,¹⁸]octadeca-7(18),8,10,15-tetraen-14-ol |
|---|
| Traditional Name | ethylmorphine |
|---|
| SMILES | [H][C@@]12OC3=C(OCC)C=CC4=C3[C@@]11CCN(C)[C@]([H])(C4)[C@]1([H])C=C[C@@H]2O |
|---|
| InChI Identifier | InChI=1S/C19H23NO3/c1-3-22-15-7-4-11-10-13-12-5-6-14(21)18-19(12,8-9-20(13)2)16(11)17(15)23-18/h4-7,12-14,18,21H,3,8-10H2,1-2H3/t12-,13+,14-,18-,19-/m0/s1 |
|---|
| InChI Key | OGDVEMNWJVYAJL-LEPYJNQMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as morphinans. These are polycyclic compounds with a four-ring skeleton with three condensed six-member rings forming a partially hydrogenated phenanthrene moiety, one of which is aromatic while the two others are alicyclic. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Morphinans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Morphinans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Morphinan
- Phenanthrene
- Tetralin
- Coumaran
- Alkyl aryl ether
- Aralkylamine
- Piperidine
- Benzenoid
- Secondary alcohol
- Tertiary amine
- Tertiary aliphatic amine
- Ether
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic nitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0671-1090000000-ee6bb48baf50c5f0670d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00fr-8019000000-beb618b0f58f701fb24e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0049000000-e64f6928d31bc2f5e107 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1095000000-59a9dd24e82a0c7408b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pvi-2090000000-b870f6f4d523dfc80ab1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0029000000-34c8963f215272a01a9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03e9-1095000000-566ee946086b9b47cb7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00os-2090000000-2f55fa4e64af44c96a21 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0009000000-879645690cd5fc806db2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0049000000-f058a1d6993a96cbdcbe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k92-0091000000-89c269fb29eb1ca16959 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-19200665d806a98057c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0039000000-ded7c070e5e8b4cb3500 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0091000000-7a013b74465d3642aeaa | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01466 |
|---|
| HMDB ID | HMDB0015509 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ethylmorphine |
|---|
| Chemspider ID | 4514250 |
|---|
| ChEBI ID | 1192901 |
|---|
| PubChem Compound ID | 5359271 |
|---|
| Kegg Compound ID | C07537 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Aasmundstad TA, Xu BQ, Johansson I, Ripel A, Bjorneboe A, Christophersen AS, Bodd E, Morland J: Biotransformation and pharmacokinetics of ethylmorphine after a single oral dose. Br J Clin Pharmacol. 1995 Jun;39(6):611-20. | | 2. Xu BQ, Aasmundstad TA, Lillekjendlie B, Bjorneboe A, Christophersen AS, Morland J: Effects of ethanol on ethylmorphine metabolism in isolated rat hepatocytes: characterization by means of a multicompartmental model. Pharmacol Toxicol. 1997 Apr;80(4):171-81. |
|
|---|