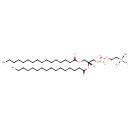
| 1,2-Bis(hexadecanoyl)-sn-glycero-3-phosphocholine | ChEBI |
| 1,2-Dipalmitoyl-L-lecithin | ChEBI |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphatidylcholine | ChEBI |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine | ChEBI |
| 1,2-Dipalmitoylphosphatidylcholine | ChEBI |
| 1-16:0-2-16:0-Phosphatidylcholine | ChEBI |
| 16:0-16:0-PC | ChEBI |
| Colfosceril palmitate | ChEBI |
| Colfoscerili palmitas | ChEBI |
| Dipalmitoyl phosphatidylcholine | ChEBI |
| Dipalmitoyl-GPC | ChEBI |
| Dipalmitoylphosphatidylcholine | ChEBI |
| GPC(16:0/16:0) | ChEBI |
| GPCho 16:0/16:0 | ChEBI |
| GPCho(16:0/16:0) | ChEBI |
| Palmitate de colfosceril | ChEBI |
| Palmitato de colfoscerilo | ChEBI |
| PC 16:0/16:0 | ChEBI |
| PC(32:0) | ChEBI |
| Phosphatidylcholine 16:0/16:0 | ChEBI |
| Phosphatidylcholine(16:0/16:0) | ChEBI |
| Phosphatidylcholine(32:0) | ChEBI |
| Colfosceril palmitic acid | Generator |
| Palmitic acid de colfosceril | Generator |
| 1,2-Dipalmitoyl-rac-glycero-3-phosphocholine | HMDB |
| Lecithin | HMDB |
| 1,2-Dihexadecanoyl-rac-glycero-3-phosphocholine | HMDB |
| GPCho(32:0) | HMDB |
| (R)-4-Hydroxy-N,N,N-trimethyl-10-oxo-7-[(1-oxohexadecyl)oxy]-3,5,9-trioxa-4-phosphapentacosan-1-aminium 4-oxide hydroxide inner salt | HMDB |
| (R)-4-Hydroxy-N,N,N-trimethyl-10-oxo-7-[(1-oxohexadecyl)oxy]-3,5,9-trioxa-4-phosphapentacosan-1-aminium 4-oxide inner salt | HMDB |
| 1,2-Bis(palmitoyl)-sn-glycero-3-phosphocholine | HMDB |
| 1,2-Dihexadecanoyl-sn-glycerol-3-phosphorylcholine | HMDB |
| 1,2-Dipalmitoyl-3-sn-phosphatidylcholine | HMDB |
| 1,2-Dipalmitoyl-L-3-phosphatidylcholine | HMDB |
| 1,2-Dipalmitoyl-L-a-lecithin | HMDB |
| 1,2-Dipalmitoyl-L-a-phosphatidylcholine | HMDB |
| 1,2-Dipalmitoyl-L-alpha-lecithin | HMDB |
| 1,2-Dipalmitoyl-L-alpha-phosphatidylcholine | HMDB |
| 1,2-Dipalmitoyl-L-phosphatidylcholine | HMDB |
| 1,2-Dipalmitoyl-sn-3-glycerophosphocholine | HMDB |
| 1,2-Dipalmitoyl-sn-glycero-3-phosphorylcholine | HMDB |
| 1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine | HMDB |
| 1,2-Dipalmitoyl-sn-glycerophosphocholine | HMDB |
| 1,2-Dipalmitoyl-sn-glycerophosphorylcholine | HMDB |
| 1,2-Dipalmitoyl-sn-glyceryl-3-phosphocholine | HMDB |
| 1,2-Dipalmitoyl-sn-phosphatidylcholine | HMDB |
| 1,2-Dipalmitoylglycero-3-phosphocholine | HMDB |
| 1,2-L-a-Dipalmitoylphosphatidylcholine | HMDB |
| 1,2-L-alpha-Dipalmitoylphosphatidylcholine | HMDB |
| b,g-Dipalmitoyl L-a-phosphatidylcholine | HMDB |
| b,g-Dipalmitoyl L-alpha-phosphatidylcholine | HMDB |
| b,g-Dipalmitoyl-L-(a)-lecithin | HMDB |
| b,g-Dipalmitoyl-L-phosphatidylcholine | HMDB |
| Dihexadecanoyl-sn-glycero-3-phosphocholine | HMDB |
| Dipalmitoyl L-a-phosphatidylcholine | HMDB |
| Dipalmitoyl L-alpha-phosphatidylcholine | HMDB |
| Dipalmitoyl-L-3-glycerylphosphorylcholine | HMDB |
| Dipalmitoyl-L-a-lecithin | HMDB |
| Dipalmitoyl-L-a-phosphatidylcholine | HMDB |
| Dipalmitoyl-L-alpha-lecithin | HMDB |
| Dipalmitoyl-L-alpha-phosphatidylcholine | HMDB |
| Dipalmitoyl-sn-3-phosphatidylcholine | HMDB |
| DPPC | HMDB |
| L-1,2-Dipalmitoyl-a-lecithin | HMDB |
| L-1,2-Dipalmitoyl-alpha-lecithin | HMDB |
| L-1,2-Dipalmitoylphosphatidylcholine | HMDB |
| L-a-1,2-Dipalmitoyl lecithin | HMDB |
| L-a-Dipalmitoylecithin | HMDB |
| L-a-Dipalmitoyllecithin | HMDB |
| L-a-Dipalmitoylphosphatidylcholine | HMDB |
| L-a-DPPC | HMDB |
| L-alpha-1,2-Dipalmitoyl lecithin | HMDB |
| L-alpha-Dipalmitoylecithin | HMDB |
| L-alpha-Dipalmitoyllecithin | HMDB |
| L-alpha-Dipalmitoylphosphatidylcholine | HMDB |
| L-alpha-DPPC | HMDB |
| L-b,g-Dipalmitoyl-a-lecithin | HMDB |
| L-b,g-Dipalmitoyl-a-phosphatidylcholine | HMDB |
| L-b,g-Dipalmitoyl-alpha-lecithin | HMDB |
| L-b,g-Dipalmitoyl-alpha-phosphatidylcholine | HMDB |
| L-b,g-Dipalmitoylphosphatidylcholine | HMDB |
| L-Dipalmitoyl lecithin | HMDB |
| L-DPPC | HMDB |
| PC Aa C32:0 | HMDB |
| sn-3-Dipalmitoyllecithin | HMDB |
| 1,2-Dipalmitoyl-GPC | HMDB |
| PC(16:0/16:0) | Lipid Annotator |
| 1,2-Distearoyl-glycero-3-phosphocholine | HMDB |