| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:52:20 UTC |
|---|
| Update Date | 2016-11-09 01:15:56 UTC |
|---|
| Accession Number | CHEM019160 |
|---|
| Identification |
|---|
| Common Name | Lumefantrine |
|---|
| Class | Small Molecule |
|---|
| Description | Lumefantrine is an antimalarial drug used in combination with artemether for the treatment of multi-drug resistant strains of falciparum malaria. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
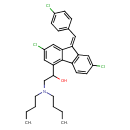
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+-)-2,7-Dichloro-9-((Z)-p-chlorobenzylidene)-alpha-((dibutylamino)methyl)fluorene-4-methanol | ChEBI | | 2-Dibutylamino-1-[2,7-dichloro-9-(4-chloro-benzylidene)-9H-fluoren-4-yl]-ethanol | ChEBI | | 2-Dibutylamino-1-{2,7-dichloro-9-[1-(4-chloro-phenyl)-meth-(Z)-ylidene]-9H-fluoren-4-yl}-ethanol | ChEBI | | Benflumetol | ChEBI | | DL-Benflumelol | ChEBI | | (+-)-2,7-Dichloro-9-((Z)-p-chlorobenzylidene)-a-((dibutylamino)methyl)fluorene-4-methanol | Generator | | (+-)-2,7-Dichloro-9-((Z)-p-chlorobenzylidene)-α-((dibutylamino)methyl)fluorene-4-methanol | Generator | | Benflumetol, (+)-isomer | HMDB | | Benflumetol, (+-)-isomer | HMDB | | Benflumetol, (-)-isomer | HMDB |
|
|---|
| Chemical Formula | C30H32Cl3NO |
|---|
| Average Molecular Mass | 528.940 g/mol |
|---|
| Monoisotopic Mass | 527.155 g/mol |
|---|
| CAS Registry Number | 82186-77-4 |
|---|
| IUPAC Name | 2-(dibutylamino)-1-[(9Z)-2,7-dichloro-9-[(4-chlorophenyl)methylidene]-9H-fluoren-4-yl]ethan-1-ol |
|---|
| Traditional Name | lumefantrine |
|---|
| SMILES | CCCCN(CCCC)CC(O)C1=C2C(=CC(Cl)=C1)\C(=C/C1=CC=C(Cl)C=C1)C1=C2C=CC(Cl)=C1 |
|---|
| InChI Identifier | InChI=1S/C30H32Cl3NO/c1-3-5-13-34(14-6-4-2)19-29(35)28-18-23(33)17-27-25(15-20-7-9-21(31)10-8-20)26-16-22(32)11-12-24(26)30(27)28/h7-12,15-18,29,35H,3-6,13-14,19H2,1-2H3/b25-15- |
|---|
| InChI Key | DYLGFOYVTXJFJP-MYYYXRDXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fluorenes. Fluorenes are compounds containing a fluorene moiety, which consists of two benzene rings connected through either a cyclopentane, cyclopentene, or cyclopenta-1,3-diene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Fluorenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Fluorenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fluorene
- Chlorobenzene
- Aralkylamine
- Halobenzene
- Aryl chloride
- Aryl halide
- Monocyclic benzene moiety
- 1,2-aminoalcohol
- Tertiary aliphatic amine
- Secondary alcohol
- Tertiary amine
- Organochloride
- Organohalogen compound
- Alcohol
- Aromatic alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Amine
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000l-5908110000-30364837a4164a8491be | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0bt9-5300960000-f57d30fd0683000ba1df | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Lumefantrine,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03fr-0000190000-2026e5ba94f08f73450d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-3309470000-dd7cd2616b9942c35f6d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9214100000-82786f2daa0ffb34e593 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000090000-75a6769f5d87d37f6531 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0602290000-621f19a6f82ad39466a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ul1-5209000000-229aedab58de48c7d7fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000090000-f3e5f6566ad7bc0d6931 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-3100290000-5859b739d14324e74b77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01q1-9301010000-442ed46841c560639476 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000090000-6f1797933f6c478c462e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0205690000-b150da224a2d334104c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f89-7509400000-ffd74bf582f10032d904 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06708 |
|---|
| HMDB ID | HMDB0015653 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Lumefantrine |
|---|
| Chemspider ID | 4941944 |
|---|
| ChEBI ID | 156095 |
|---|
| PubChem Compound ID | 6437380 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|