| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:51:07 UTC |
|---|
| Update Date | 2016-11-09 01:15:56 UTC |
|---|
| Accession Number | CHEM019138 |
|---|
| Identification |
|---|
| Common Name | Halobetasol propionate |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
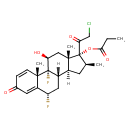
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ulobetasol propionate | Kegg | | Ultravate | Kegg | | Ulobetasol propionic acid | Generator | | Ultravic acid | Generator | | (1R,2S,8S,10S,11S,13S,14R,15S,17S)-14-(2-Chloroacetyl)-1,8-difluoro-17-hydroxy-2,13,15-trimethyl-5-oxotetracyclo[8.7.0.0,.0,]heptadeca-3,6-dien-14-yl propanoic acid | Generator | | [(6S,8S,9R,10S,11S,13S,14S,16S,17R)-17-(2-Chloroacetyl)-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] propanoic acid | Generator | | 6-Fluoroclobetasol 17-propionate | MeSH | | Halobetasol propionate | MeSH | | Ulobetasol | MeSH | | Halobetasol | MeSH | | 6 alpha-Fluoroclobetasol 17-propionate | MeSH | | HALOBETASOL propionic acid | Generator |
|
|---|
| Chemical Formula | C25H31ClF2O5 |
|---|
| Average Molecular Mass | 484.960 g/mol |
|---|
| Monoisotopic Mass | 484.183 g/mol |
|---|
| CAS Registry Number | 66852-54-8 |
|---|
| IUPAC Name | (1R,2S,8S,10S,11S,13S,14R,15S,17S)-14-(2-chloroacetyl)-1,8-difluoro-17-hydroxy-2,13,15-trimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-3,6-dien-14-yl propanoate |
|---|
| Traditional Name | (1R,2S,8S,10S,11S,13S,14R,15S,17S)-14-(2-chloroacetyl)-1,8-difluoro-17-hydroxy-2,13,15-trimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-3,6-dien-14-yl propanoate |
|---|
| SMILES | [H][C@@]12C[C@H](C)[C@](OC(=O)CC)(C(=O)CCl)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])C[C@H](F)C2=CC(=O)C=C[C@]12C |
|---|
| InChI Identifier | InChI=1S/C25H31ClF2O5/c1-5-21(32)33-25(20(31)12-26)13(2)8-15-16-10-18(27)17-9-14(29)6-7-22(17,3)24(16,28)19(30)11-23(15,25)4/h6-7,9,13,15-16,18-19,30H,5,8,10-12H2,1-4H3/t13-,15-,16-,18-,19-,22-,23-,24-,25-/m0/s1 |
|---|
| InChI Key | BDSYKGHYMJNPAB-LICBFIPMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- Steroid ester
- Hydroxysteroid
- Halo-steroid
- 6-halo-steroid
- 9-halo-steroid
- Oxosteroid
- 3-oxosteroid
- 11-hydroxysteroid
- 11-beta-hydroxysteroid
- 3-oxo-delta-1,4-steroid
- Delta-1,4-steroid
- Alpha-acyloxy ketone
- Alpha-haloketone
- Cyclic alcohol
- Alpha-chloroketone
- Carboxylic acid ester
- Cyclic ketone
- Fluorohydrin
- Secondary alcohol
- Halohydrin
- Ketone
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Alcohol
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Alkyl halide
- Organofluoride
- Organochloride
- Alkyl chloride
- Organohalogen compound
- Alkyl fluoride
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05n0-2001900000-9ed7031acf84122f3b96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9035700000-e3f8b8fb405e006f6536 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-3069000000-dd106a45fb3273b4e4d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-2000900000-34a67b1506dccd0761c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-3003900000-91cac352cac72606446c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-9106300000-761683f52d3f8f6a6f66 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT001867 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6918178 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|