| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:50:04 UTC |
|---|
| Update Date | 2016-11-09 01:15:56 UTC |
|---|
| Accession Number | CHEM019116 |
|---|
| Identification |
|---|
| Common Name | Trifluridine |
|---|
| Class | Small Molecule |
|---|
| Description | Trifluridine is a fluorinated pyrimidine nucleoside that is structurally related to . It is an active antiviral agent in ophthalmic solutions used mainly in the treatment of primary keratoconjunctivitis and recurrent epithelial keratitis due to herpes simplex virus. It displays effective antiviral activity against Herpes simplex virus type 1 and 2 .
The combination product of trifluridine with tipiracil marketed as Lonsurf has been approved in Japan, the United States, and the European Union for the treatment of adult patients with metastatic colorectal cancer who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF biological therapy, and if RAS wild-type, an anti-EGFR therapy. In the anticancer therapy, trifluridine acts as a thymidine-based nucleoside metabolic inhibitor that gets incorporated into DNA of cancer cells following cell uptake to aberrate DNA function during cell replication . |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
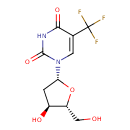
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-(Trifluoromethyl)deoxyuridine | ChEBI | | 5-Trifluoromethyl-2-deoxyuridine | ChEBI | | Trifluoromethyldeoxyuridine | ChEBI | | Trifluorothymidine | ChEBI | | Trifluorothymine deoxyriboside | ChEBI | | Trifluridina | ChEBI | | Trifluridinum | ChEBI | | Viroptic | ChEBI | | Elcon brand OF trifluridine | HMDB | | Viromidin | HMDB | | 5-Trifluoromethyl-2'-deoxyuridine | HMDB | | Allergan brand OF trifluridine | HMDB | | Mann brand OF trifluridine | HMDB | | Monarch brand OF trifluridine | HMDB | | Tramedico brand OF trifluridine | HMDB | | Trifluoridine | HMDB | | 2'-Deoxy-5-(trifluoromethyl)uridine | HMDB | | 5 Trifluoromethyl 2' deoxyuridine | HMDB | | TFT Ophtiole | HMDB | | Triflumann | HMDB | | Virophta | HMDB |
|
|---|
| Chemical Formula | C10H11F3N2O5 |
|---|
| Average Molecular Mass | 296.200 g/mol |
|---|
| Monoisotopic Mass | 296.062 g/mol |
|---|
| CAS Registry Number | 70-00-8 |
|---|
| IUPAC Name | 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-(trifluoromethyl)-1,2,3,4-tetrahydropyrimidine-2,4-dione |
|---|
| Traditional Name | trifluridine |
|---|
| SMILES | OC[C@H]1O[C@H](C[C@@H]1O)N1C=C(C(=O)NC1=O)C(F)(F)F |
|---|
| InChI Identifier | InChI=1S/C10H11F3N2O5/c11-10(12,13)4-2-15(9(19)14-8(4)18)7-1-5(17)6(3-16)20-7/h2,5-7,16-17H,1,3H2,(H,14,18,19)/t5-,6+,7+/m0/s1 |
|---|
| InChI Key | VSQQQLOSPVPRAZ-RRKCRQDMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrimidine 2'-deoxyribonucleosides. Pyrimidine 2'-deoxyribonucleosides are compounds consisting of a pyrimidine linked to a ribose which lacks a hydroxyl group at position 2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Pyrimidine nucleosides |
|---|
| Sub Class | Pyrimidine 2'-deoxyribonucleosides |
|---|
| Direct Parent | Pyrimidine 2'-deoxyribonucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidine 2'-deoxyribonucleoside
- Pyrimidone
- Hydroxypyrimidine
- Hydropyrimidine
- Pyrimidine
- Tetrahydrofuran
- Heteroaromatic compound
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Organic nitrogen compound
- Alkyl halide
- Alkyl fluoride
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-9070000000-5b0bc8f23d4e75143939 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0v70-9845200000-37084cd9faa19fab8cdd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-98b3bdac019c141f7f2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0910000000-88c39b37d01c15092d5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001r-1900000000-6af805305211581c822e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0w2a-1490000000-06d5a9ab5df20c112030 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-0790000000-aca1588a5498c0e36172 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-5900000000-3142b258f9393903ca54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002b-0090000000-9941fbdce182b1697ae3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ufr-1920000000-0b7d290fcb81239751b5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9500000000-c59f899250e30f03cf63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0290000000-d2c6f13535b38e719f66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0910000000-2f204a289423b2c2048d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-2960000000-d74ea6cb75e5abd92cdb | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00432 |
|---|
| HMDB ID | HMDB0014576 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Trifluridine |
|---|
| Chemspider ID | 6020 |
|---|
| ChEBI ID | 75179 |
|---|
| PubChem Compound ID | 6256 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. O'Brien WJ, Taylor JL: Therapeutic response of herpes simplex virus-induced corneal edema to trifluridine in combination with immunosuppressive agents. Invest Ophthalmol Vis Sci. 1991 Aug;32(9):2455-61. | | 2. Kuster P, Taravella M, Gelinas M, Stepp P: Delivery of trifluridine to human cornea and aqueous using collagen shields. CLAO J. 1998 Apr;24(2):122-4. | | 3. Costin D, Dogaru M, Popa AS, Cijevschi I: [Trifluridine therapy in herpetic in keratitis]. Rev Med Chir Soc Med Nat Iasi. 2004 Apr-Jun;108(2):409-12. | | 4. Hong DS, Abbruzzese JL, Bogaard K, Lassere Y, Fukushima M, Mita A, Kuwata K, Hoff PM: Phase I study to determine the safety and pharmacokinetics of oral administration of TAS-102 in patients with solid tumors. Cancer. 2006 Sep 15;107(6):1383-90. | | 5. Temmink OH, Prins HJ, van Gelderop E, Peters GJ: The Hollow Fibre Assay as a model for in vivo pharmacodynamics of fluoropyrimidines in colon cancer cells. Br J Cancer. 2007 Jan 15;96(1):61-6. Epub 2006 Dec 19. | | 6. Overman MJ, Kopetz S, Varadhachary G, Fukushima M, Kuwata K, Mita A, Wolff RA, Hoff P, Xiong H, Abbruzzese JL: Phase I clinical study of three times a day oral administration of TAS-102 in patients with solid tumors. Cancer Invest. 2008 Oct;26(8):794-9. doi: 10.1080/07357900802087242. | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=19816940 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=19886911 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=20861476 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=21278209 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=21491084 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=22076553 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=22607463 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=22662200 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=22735906 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=22977515 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=23343513 | | 18. https://www.ncbi.nlm.nih.gov/pubmed/?term=23386782 | | 19. https://www.ncbi.nlm.nih.gov/pubmed/?term=23521072 | | 20. https://www.ncbi.nlm.nih.gov/pubmed/?term=23919755 |
|
|---|