| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:46:32 UTC |
|---|
| Update Date | 2016-11-09 01:15:55 UTC |
|---|
| Accession Number | CHEM019039 |
|---|
| Identification |
|---|
| Common Name | Valrubicin |
|---|
| Class | Small Molecule |
|---|
| Description | Valrubicin (N-trifluoroacetyladriamycin-14-valerate) is a chemotherapy drug commonly marketed under the trade name VALSTAR. It is a semisynthetic analog of the , which is an anthracycline drug. Used in the treatment of the bladder cancer, valrubicin is administered by direct infusion into the bladder. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
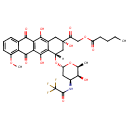
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| AD-32valrubicin | ChEMBL | | Valstar | ChEMBL, MeSH | | [2-oxo-2-[(2S,4S)-2,5,12-Trihydroxy-4-[(2R,4S,5S,6S)-5-hydroxy-6-methyl-4-[(2,2,2-trifluoroacetyl)amino]oxan-2-yl]oxy-7-methoxy-6,11-dioxo-3,4-dihydro-1H-tetracen-2-yl]ethyl] pentanoic acid | Generator | | Valrubicin | MeSH | | AD-32 | MeSH | | N-Trifluoroacetyladriamycin 14-valerate | MeSH | | Paladin brand OF valrubicin | MeSH | | Valtaxin | MeSH | | AD 32 | MeSH | | 2,2,2-Trifluoro-N-[(2S,3S,4S,6R)-3-hydroxy-2-methyl-6-{[(1S,3S)-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-3-[2-(pentanoyloxy)acetyl]-1,2,3,4,6,11-hexahydrotetracen-1-yl]oxy}oxan-4-yl]ethanimidate | Generator |
|
|---|
| Chemical Formula | C34H36F3NO13 |
|---|
| Average Molecular Mass | 723.651 g/mol |
|---|
| Monoisotopic Mass | 723.214 g/mol |
|---|
| CAS Registry Number | 56124-62-0 |
|---|
| IUPAC Name | 2-oxo-2-[(2S,4S)-2,5,12-trihydroxy-4-{[(2R,4S,5S,6S)-5-hydroxy-6-methyl-4-(2,2,2-trifluoroacetamido)oxan-2-yl]oxy}-7-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-2-yl]ethyl pentanoate |
|---|
| Traditional Name | 2-oxo-2-[(2S,4S)-2,5,12-trihydroxy-4-{[(2R,4S,5S,6S)-5-hydroxy-6-methyl-4-(2,2,2-trifluoroacetamido)oxan-2-yl]oxy}-7-methoxy-6,11-dioxo-3,4-dihydro-1H-tetracen-2-yl]ethyl pentanoate |
|---|
| SMILES | [H][C@@]1(C[C@@](O)(CC2=C(O)C3=C(C(O)=C12)C(=O)C1=C(OC)C=CC=C1C3=O)C(=O)COC(=O)CCCC)O[C@H]1C[C@H](NC(=O)C(F)(F)F)[C@H](O)[C@H](C)O1 |
|---|
| InChI Identifier | InChI=1S/C34H36F3NO13/c1-4-5-9-21(40)49-13-20(39)33(47)11-16-24(19(12-33)51-22-10-17(27(41)14(2)50-22)38-32(46)34(35,36)37)31(45)26-25(29(16)43)28(42)15-7-6-8-18(48-3)23(15)30(26)44/h6-8,14,17,19,22,27,41,43,45,47H,4-5,9-13H2,1-3H3,(H,38,46)/t14-,17-,19-,22-,27+,33-/m0/s1 |
|---|
| InChI Key | ZOCKGBMQLCSHFP-KQRAQHLDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pteridines and derivatives. These are polycyclic aromatic compounds containing a pteridine moiety, which consists of a pyrimidine fused to a pyrazine ring to form pyrimido(4,5-b)pyrazine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Pteridines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pteridine
- Aminopyrazine
- Aminopyrimidine
- Monocyclic benzene moiety
- Pyrazine
- Pyrimidine
- Benzenoid
- Imidolactam
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Hydrocarbon derivative
- Primary amine
- Organonitrogen compound
- Organopnictogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05gr-5114715900-82fd266e174de2f97209 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000b-6127901100-267c59a2d37c5997aa26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052e-9404200000-65b3255f331b525361f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ul0-8900313600-bbece7f4abec6a7b5a2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-6913101100-4918f0c2534530b35410 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-4900300000-32fb9315708051f20bcf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00385 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Valrubicin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 454216 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|