| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:45:55 UTC |
|---|
| Update Date | 2016-11-09 01:15:55 UTC |
|---|
| Accession Number | CHEM019026 |
|---|
| Identification |
|---|
| Common Name | Prulifloxacin |
|---|
| Class | Small Molecule |
|---|
| Description | Prulifloxacin has been investigated for the treatment of Urinary Tract Infection. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
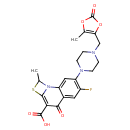
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Prulifloxacin | MeSH | | 6-fluoro-1-Methyl-7-(4-(5-methyl-2-oxo-1,3-dioxelen-4-yl)methyl-1-piperazinyl)-4-oxo-4H-(1,3)thiazeto(3,2-a)quinoline-3-carboxylic acid | MeSH | | 6-Fluoro-1-methyl-7-{4-[(5-methyl-2-oxo-2H-1,3-dioxol-4-yl)methyl]piperazin-1-yl}-4-oxo-1H,4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate | Generator |
|
|---|
| Chemical Formula | C21H20FN3O6S |
|---|
| Average Molecular Mass | 461.460 g/mol |
|---|
| Monoisotopic Mass | 461.106 g/mol |
|---|
| CAS Registry Number | 123447-62-1 |
|---|
| IUPAC Name | 6-fluoro-1-methyl-7-{4-[(5-methyl-2-oxo-2H-1,3-dioxol-4-yl)methyl]piperazin-1-yl}-4-oxo-1H,4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic acid |
|---|
| Traditional Name | prulifloxacin |
|---|
| SMILES | CC1SC2=C(C(O)=O)C(=O)C3=CC(F)=C(C=C3N12)N1CCN(CC2=C(C)OC(=O)O2)CC1 |
|---|
| InChI Identifier | InChI=1S/C21H20FN3O6S/c1-10-16(31-21(29)30-10)9-23-3-5-24(6-4-23)15-8-14-12(7-13(15)22)18(26)17(20(27)28)19-25(14)11(2)32-19/h7-8,11H,3-6,9H2,1-2H3,(H,27,28) |
|---|
| InChI Key | PWNMXPDKBYZCOO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as quinoline carboxylic acids. These are quinolines in which the quinoline ring system is substituted by a carboxyl group at one or more positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Quinoline carboxylic acids |
|---|
| Direct Parent | Quinoline carboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Quinoline-3-carboxylic acid
- Fluoroquinolone
- N-arylpiperazine
- Aminoquinoline
- Dihydroquinolone
- Haloquinoline
- Dihydroquinoline
- Pyridine carboxylic acid
- Pyridine carboxylic acid or derivatives
- Aryl thioether
- Tertiary aliphatic/aromatic amine
- Dialkylarylamine
- N-alkylpiperazine
- Aralkylamine
- Alkylarylthioether
- 1,4-diazinane
- Piperazine
- Carbonic acid diester
- Pyridine
- Aryl halide
- Aryl fluoride
- Benzenoid
- Vinylogous thioester
- Heteroaromatic compound
- Vinylogous amide
- Amino acid
- Tertiary aliphatic amine
- Amino acid or derivatives
- Tertiary amine
- Oxacycle
- Azacycle
- Thioether
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Amine
- Organooxygen compound
- Organic nitrogen compound
- Organic oxide
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organohalogen compound
- Organofluoride
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02t9-1119300000-35303584848d645707bb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0004900000-eb80d2b212211d8b1a2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-3009400000-8929937e77f03ff603cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0imr-0469000000-bf052dd96feb752f2156 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0002900000-533379839e37e22ff3a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-2009100000-8ef9b3efd23bacd8ca6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bt9-9001000000-dae469c374d3bc953bc5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB11892 |
|---|
| HMDB ID | HMDB0256881 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Prulifloxacin |
|---|
| Chemspider ID | 59351 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|