| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:41:08 UTC |
|---|
| Update Date | 2016-11-09 01:15:53 UTC |
|---|
| Accession Number | CHEM018939 |
|---|
| Identification |
|---|
| Common Name | Tanespimycin |
|---|
| Class | Small Molecule |
|---|
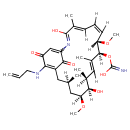
| Description | A 19-membered macrocyle that is geldanamycin in which the methoxy substituent attached to the benzoquinone moiety has been replaced by an allylamino group. It is a potent inhibitor of heat shock protein 90 (Hsp90). A less toxic analogue than geldanamycin, it induces apoptosis and displays antitumour effects. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 17-(Allylamino)-17-demethoxygeldanamycin | ChEBI | | 17-(Allylamino)geldanamycin | ChEBI | | 17-AAG | ChEBI | | 17-Allylaminogeldanamycin | ChEBI | | 17-Demethoxy-17-(2-propenylamino)geldanamycin | ChEBI | | 17-Demethoxy-17-allylamino geldanamycin | ChEBI | | 17-N-Allylamino-17-demethoxygeldanamycin | ChEBI | | 17AAG | ChEBI | | NSC 330507 | ChEBI | | NSC-330507 | ChEBI | | Tanespimycina | ChEBI | | Tanespimycine | ChEBI | | Tanespimycinum | ChEBI | | 17-Allylamino-17-demethoxygeldanamycin hydroquinone salt | MeSH | | IPI-504 | MeSH | | IPI-493 | MeSH | | 17-Allylamino-17-demethoxygeldanamycin hydroquinone | MeSH | | 17-(Allylamino)-17-demethoxy-geldanamycin | MeSH | | IPI 504 | MeSH | | IPI 493 | MeSH | | 17-Allyl-aminogeldanamycin | MeSH | | 17-Allylamino-geldanamycin | MeSH | | 17-Allylamino-17-demethoxygeldanamycin | MeSH | | Retaspimycin hydrochloride | MeSH |
|
|---|
| Chemical Formula | C31H43N3O8 |
|---|
| Average Molecular Mass | 585.698 g/mol |
|---|
| Monoisotopic Mass | 585.305 g/mol |
|---|
| CAS Registry Number | 75747-14-7 |
|---|
| IUPAC Name | {[(4E,6Z,8S,9S,10E,12S,13R,14S,16R)-3,13-dihydroxy-8,14-dimethoxy-4,10,12,16-tetramethyl-20,22-dioxo-19-[(prop-2-en-1-yl)amino]-2-azabicyclo[16.3.1]docosa-1(21),2,4,6,10,18-hexaen-9-yl]oxy}methanimidic acid |
|---|
| Traditional Name | AAG |
|---|
| SMILES | [H]/C1=C([H])/[C@]([H])(OC)[C@@]([H])(OC(O)=N)C(C)=C([H])[C@]([H])(C)[C@@]([H])(O)[C@]([H])(C[C@]([H])(C)CC2=C(NCC=C)C(=O)C=C(N=C(O)\C(C)=C\1/[H])C2=O)OC |
|---|
| InChI Identifier | InChI=1S/C31H43N3O8/c1-8-12-33-26-21-13-17(2)14-25(41-7)27(36)19(4)15-20(5)29(42-31(32)39)24(40-6)11-9-10-18(3)30(38)34-22(28(21)37)16-23(26)35/h8-11,15-17,19,24-25,27,29,33,36H,1,12-14H2,2-7H3,(H2,32,39)(H,34,38)/b11-9-,18-10+,20-15+/t17-,19+,24+,25+,27-,29+/m1/s1 |
|---|
| InChI Key | AYUNIORJHRXIBJ-TXHRRWQRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as macrolactams. These are cyclic amides of amino carboxylic acids, having a 1-azacycloalkan-2-one structure, or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. They are nitrogen analogues (the a nitrogen atom replacing the o atom of the cyclic carboxylic acid group ) of the naturally occurring macrolides. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Macrolactams |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Macrolactams |
|---|
| Alternative Parents | |
|---|
| Substituents | - Macrolactam
- Vinylogous amide
- Carbamic acid ester
- Amino acid or derivatives
- Carboxamide group
- Ketone
- Lactam
- Carbonic acid derivative
- Secondary alcohol
- Cyclic ketone
- Secondary carboxylic acid amide
- Carboxylic acid derivative
- Dialkyl ether
- Secondary aliphatic amine
- Enamine
- Ether
- Azacycle
- Organoheterocyclic compound
- Secondary amine
- Carbonyl group
- Organic nitrogen compound
- Amine
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00ko-2000090000-80400e0f67a908654e82 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-4000090000-076b30aaab45634e1e34 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9121130000-7b50f261b5e13fe91c96 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-8000090000-d2c9ad0c41cbdaf1ae18 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000060000-0790f5184b74b931c570 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9001100000-cb71a91c12b9c2590d40 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB05134 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 17-N-Allylamino-17-demethoxygeldanamycin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 64153 |
|---|
| PubChem Compound ID | 6505803 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|