| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:40:55 UTC |
|---|
| Update Date | 2016-11-09 01:15:53 UTC |
|---|
| Accession Number | CHEM018931 |
|---|
| Identification |
|---|
| Common Name | Eltanolone |
|---|
| Class | Small Molecule |
|---|
| Description | As of March 2019, brexanolone - developed and made available commercially by Sage Therapeutics Inc. as the brand name product Allopregnanolone - is the first drug to have ever been approved by the US FDA specifically for the treatment of postpartum depression (PPD) in adult females . Since PPD, like various other types of depression, is characterized by feelings of sadness, worthlessness or guilt, cognitive impairment, and/or possibly suicidal ideation, it is considered a life-threatening condition . Studies have consequently found that PPD can genuinely have profound negative effects on the maternal-infant bond and later infant development . The development and availability of brexanolone for the treatment of PPD in adult females subsequently provides a new and promising therapy where few existed before .
In particular, the use of brexanolone in treating PPD is surrounded with promise because it acts in part as a synthetic supplement for possible deficiencies in endogenous brexanolone (allopregnanolone) in postpartum women susceptible to PPD whereas many commonly used anti-depressive medications elicit actions that may modulate the presence and activity of substances like serotonin, norepinephrine, and/or monoamine oxidase but do not mediate activities directly associated with PPD like natural fluctuations in the levels of endogenous neuroactive steroids like allopregnanolone .
And finally, although brexanolone may also be undergoing clinical trials to investigate its abilities to treat super-refractory status epilepticus, it appears that some such studies have failed to meet primary endpoints that compare success in the weaning of third-line agents and resolution of potentially life-threatening status epilepticus with brexanolone vs. placebo when added to standard-of-care . |
|---|
| Contaminant Sources | - FooDB Chemicals
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
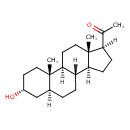
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3alpha,5alpha)-3-Hydroxypregnan-20-one | ChEBI | | 3alpha,5alpha-Tetrahydroprogesterone | ChEBI | | 3alpha,5alpha-THP | ChEBI | | 3alpha-Hydroxy-5alpha-dihydroprogesterone | ChEBI | | 3alpha-Hydroxy-5alpha-pregnan-20-one | ChEBI | | 3alpha-OH DHP | ChEBI | | 5alpha-Pregnan-3alpha-ol-20-one | ChEBI | | Allopregnan-3alpha-ol-20-one | ChEBI | | Allotetrahydroprogesterone | ChEBI | | Brexanolona | ChEBI | | Brexanolonum | ChEBI | | SAGE-547 | ChEBI | | SGE-102 | ChEBI | | Zulresso | ChEBI | | (3a,5a)-3-Hydroxypregnan-20-one | Generator | | (3Α,5α)-3-hydroxypregnan-20-one | Generator | | 3a,5a-Tetrahydroprogesterone | Generator | | 3Α,5α-tetrahydroprogesterone | Generator | | 3a,5a-THP | Generator | | 3Α,5α-THP | Generator | | 3a-Hydroxy-5a-dihydroprogesterone | Generator | | 3Α-hydroxy-5α-dihydroprogesterone | Generator | | 3a-Hydroxy-5a-pregnan-20-one | Generator | | 3Α-hydroxy-5α-pregnan-20-one | Generator | | 3a-OH DHP | Generator | | 3Α-OH DHP | Generator | | 5a-Pregnan-3a-ol-20-one | Generator | | 5Α-pregnan-3α-ol-20-one | Generator | | Allopregnan-3a-ol-20-one | Generator | | Allopregnan-3α-ol-20-one | Generator | | (+)-3a-Hydroxy-5a-pregnan-20-one | HMDB | | (3a)-Allopregnanolone | HMDB | | 3-a-Tetrahydroprogesterone | HMDB | | 3-alpha-Tetrahydroprogesterone | HMDB | | 3a,5a-Pregnanolone | HMDB | | 3a-Hydroxy-5a-pregnane-20-one | HMDB | | 5a-Pregnane-3a-ol-20-one | HMDB | | 3-Hydroxypregnan-20-one | HMDB | | alpha-Hydroxy-5 alpha-pregnan-20-one, 3 | HMDB | | Pregnan-3alpha-ol-20-one | HMDB | | Pregnanolone, (3alpha)-isomer | HMDB | | 3 Hydroxypregnan 20 one | HMDB | | 3 alpha Hydroxy 5 alpha pregnan 20 one | HMDB | | 3 alpha-Hydroxy-5 alpha-pregnan-20-one | HMDB | | Pregnan 3alpha ol 20 one | HMDB | | alpha-Pregnan-20-one, 3 alpha-hydroxy-5 | HMDB | | (+)-3alpha-Hydroxy-5alpha-pregnan-20-one | HMDB | | (+)-3Α-hydroxy-5α-pregnan-20-one | HMDB | | (3alpha)-Allopregnanolone | HMDB | | (3Α)-allopregnanolone | HMDB | | 3alpha,5alpha-Pregnanolone | HMDB | | 3alpha-Hydroxy-5alpha-pregnane-20-one | HMDB | | 3Α,5α-pregnanolone | HMDB | | 3Α-hydroxy-5α-pregnane-20-one | HMDB | | 5alpha-Pregnane-3alpha-ol-20-one | HMDB | | 5Α-pregnane-3α-ol-20-one | HMDB | | Allopregnanolone | MeSH, ChEBI |
|
|---|
| Chemical Formula | C21H34O2 |
|---|
| Average Molecular Mass | 318.494 g/mol |
|---|
| Monoisotopic Mass | 318.256 g/mol |
|---|
| CAS Registry Number | 128-20-1 |
|---|
| IUPAC Name | 1-[(1S,2S,5R,7S,10R,11S,14S,15S)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]ethan-1-one |
|---|
| Traditional Name | 1-[(1S,2S,5R,7S,10R,11S,14S,15S)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]ethanone |
|---|
| SMILES | [H][C@@]12CC[C@H](C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C[C@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16-,17+,18-,19-,20-,21+/m0/s1 |
|---|
| InChI Key | AURFZBICLPNKBZ-SYBPFIFISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- 3-hydroxysteroid
- Hydroxysteroid
- 3-alpha-hydroxysteroid
- Oxosteroid
- Cyclic alcohol
- Ketone
- Secondary alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - 3-hydroxy-5alpha-pregnan-20-one (CHEBI:50169 )
- C21 steroids (gluco/mineralocorticoids, progestogins) and derivatives (LMST02030156 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-0udi-6910000000-837b37f206729753b640 | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0udi-6910000000-837b37f206729753b640 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-1174-0292000000-fb2c0f3a1cfddb10cb08 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-004i-2139000000-ab8a6de8f5a129af086e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0129000000-90e5fcb36022dd1ba688 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-0396000000-4c39fe7ccd8e0c86e179 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-2490000000-7ac55935cc0d4da95860 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0029000000-fa09e69c9c0b6736d2e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0069000000-d1d57e3e1a99da4ed11f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zg0-1193000000-44d1597a59ac11f16a41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gb9-0019000000-5b53b3ba37ccafca449f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0lgi-1797000000-c11629ffa10cac4be118 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4m-8910000000-2813a600e98ff5997275 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-bbaa566ea695ae62f84e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0029000000-ac1c6a15538469151b6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ks-0093000000-db70f670eb2440b743f9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB11859 |
|---|
| HMDB ID | HMDB0001449 |
|---|
| FooDB ID | FDB022630 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Allopregnanolone |
|---|
| Chemspider ID | 83760 |
|---|
| ChEBI ID | 50169 |
|---|
| PubChem Compound ID | 92786 |
|---|
| Kegg Compound ID | C13712 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | M2MDB005177 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Wiebe, J. P.; Deline, C.; Buckingham, K. D.; Dave, Vinod; Stothers, J. B. Synthesis of the allylic gonadal steroids, 3a-hydroxy-4-pregnen-20-one and 3a-hydroxy-4-androsten-17-one, and of 3a-hydroxy-5a-pregnan-20-one. Steroids (1985), 45(1), 39-51. | | 2. Wiebe, J. P.; Deline, C.; Buckingham, K. D.; Dave, Vinod; Stothers, J. B. Synthesis of the allylic gonadal steroids, 3a-hydroxy-4-pregnen-20-one and 3a-hydroxy-4-androsten-17-one, and of 3a-hydroxy-5a-pregnan-20-one. Steroids (1985), 45(1), 39-51. | | 3. Kim YS, Zhang H, Kim HY: Profiling neurosteroids in cerebrospinal fluids and plasma by gas chromatography/electron capture negative chemical ionization mass spectrometry. Anal Biochem. 2000 Jan 15;277(2):187-95. | | 4. Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A: Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3239-44. | | 5. Pearson Murphy BE, Steinberg SI, Hu FY, Allison CM: Neuroactive ring A-reduced metabolites of progesterone in human plasma during pregnancy: elevated levels of 5 alpha-dihydroprogesterone in depressed patients during the latter half of pregnancy. J Clin Endocrinol Metab. 2001 Dec;86(12):5981-7. | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=10625505 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=11739473 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=19290593 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=28370307 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=28619476 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=28666923 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=28779545 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=29453777 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=29636114 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=30177236 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=30362283 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=30481569 | | 18. https://www.ncbi.nlm.nih.gov/pubmed/?term=30701996 | | 19. https://www.ncbi.nlm.nih.gov/pubmed/?term=9501247 |
|
|---|