| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:34:10 UTC |
|---|
| Update Date | 2016-11-09 01:15:52 UTC |
|---|
| Accession Number | CHEM018829 |
|---|
| Identification |
|---|
| Common Name | Mosapride citrate |
|---|
| Class | Small Molecule |
|---|
| Description | Mosapride is a gastroprokinetic agent that acts as a selective 5HT4 agonist. The major active metabolite of mosapride, known as M1, additionally acts as a 5HT3 antagonist, which accelerates gastric emptying throughout the whole of the gastrointestinal tract in humans, and is used for the treatment of gastritis, gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome. It is recommended to be taken on an empty stomach (i.e. at least one hour before food or two hours after food).In addition to its prokinetic properties, mosapride also exerts anti-inflammatory effects on the gastrointestinal tract which may contribute to some of its therapeutic effects. Mosapride also promotes neurogenesis in the gastrointestinal tract which may prove useful in certain bowel disorders. The neurogenesis is due to mosapride's effect on the 5-HT4 receptor where it acts as an agonist.Its common side effects include dry mouth, abdominal pain, dizziness, headache, insomnia, malaise, nausea, diarrhea and sometimes constipation. Unlike some other prokinetic agents, mosapride has little effect on potassium channels, no effect on hERG transfected cells, and no effect on cardiovascular function that could be detected in tests on humans. Due to the pharmacokinetics of mosapride, it would take 1,000–3,000 times the therapeutic dose to elicit cardiovascular effects. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
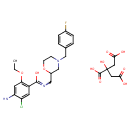
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Mosapride citric acid | Generator | | Mosapride | MeSH | | Mosapride ui05MSP015CT | MeSH | | 4-Amino-5-chloro-2-ethoxy-N-((4-(4-fluorobenzyl)-2-morpholinyl)methyl)benzamide | MeSH | | Mosapride citrate | MeSH | | 2-Hydroxypropane-1,2,3-tricarboxylate | | | 4-amino-5-chloro-2-ethoxy-N-({4-[(4-fluorophenyl)methyl]morpholin-2-yl}methyl)benzene-1-carboximidate | | | 2-Hydroxypropane-1,2,3-tricarboxylate; 4-amino-5-chloro-2-ethoxy-N-({4-[(4-fluorophenyl)methyl]morpholin-2-yl}methyl)benzene-1-carboximidate | Generator |
|
|---|
| Chemical Formula | C27H33ClFN3O10 |
|---|
| Average Molecular Mass | 614.020 g/mol |
|---|
| Monoisotopic Mass | 613.184 g/mol |
|---|
| CAS Registry Number | 112885-42-4 |
|---|
| IUPAC Name | 2-hydroxypropane-1,2,3-tricarboxylic acid; 4-amino-5-chloro-2-ethoxy-N-({4-[(4-fluorophenyl)methyl]morpholin-2-yl}methyl)benzene-1-carboximidic acid |
|---|
| Traditional Name | citric acid; mosapride |
|---|
| SMILES | OC(=O)CC(O)(CC(O)=O)C(O)=O.CCOC1=C(C=C(Cl)C(N)=C1)C(O)=NCC1CN(CC2=CC=C(F)C=C2)CCO1 |
|---|
| InChI Identifier | InChI=1S/C21H25ClFN3O3.C6H8O7/c1-2-28-20-10-19(24)18(22)9-17(20)21(27)25-11-16-13-26(7-8-29-16)12-14-3-5-15(23)6-4-14;7-3(8)1-6(13,5(11)12)2-4(9)10/h3-6,9-10,16H,2,7-8,11-13,24H2,1H3,(H,25,27);13H,1-2H2,(H,7,8)(H,9,10)(H,11,12) |
|---|
| InChI Key | HUZTYZBFZKRPFG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tricarboxylic acids and derivatives. These are carboxylic acids containing exactly three carboxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Tricarboxylic acids and derivatives |
|---|
| Direct Parent | Tricarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aminophenyl ether
- Tricarboxylic acid or derivatives
- Phenoxy compound
- Phenol ether
- Phenylmethylamine
- Benzylamine
- Aniline or substituted anilines
- Alkyl aryl ether
- Halobenzene
- Fluorobenzene
- Aralkylamine
- Chlorobenzene
- Morpholine
- Oxazinane
- Alpha-hydroxy acid
- Monocyclic benzene moiety
- Aryl halide
- Benzenoid
- Aryl fluoride
- Hydroxy acid
- Aryl chloride
- Tertiary alcohol
- Tertiary amine
- Tertiary aliphatic amine
- Oxacycle
- Azacycle
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid
- Organoheterocyclic compound
- Dialkyl ether
- Ether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Organic nitrogen compound
- Primary amine
- Organooxygen compound
- Organohalogen compound
- Organochloride
- Organofluoride
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00dj-1920400000-661fa2874767aad68d12 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00dj-2910000000-0cf829215035f4f15409 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000009000-4fae3fa34ad0cb8b7224 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000009000-4fae3fa34ad0cb8b7224 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0000009000-4fae3fa34ad0cb8b7224 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000009000-e750f76dc7ef3027218f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0000009000-e750f76dc7ef3027218f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0000009000-e750f76dc7ef3027218f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT002047 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Mosapride |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 119583 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|