Pravadoline (CHEM018756)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-05-22 05:30:38 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2016-11-09 01:15:52 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | CHEM018756 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Pravadoline | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Pravadoline (WIN 48,098) is an antiinflammatory and analgesic drug with an IC50 of 4.9 µM and a Ki of 2511 nM at CB1, related in structure to nonsteroidal anti-inflammatory drugs (NSAIDs) such as indometacin. It was developed in the 1980s as a new antiinflammatory and prostaglandin synthesis inhibitor, acting through inhibition of the enzyme cyclooxygenase (COX). However, pravadoline was found to exhibit unexpectedly strong analgesic effects, which appeared at doses ten times smaller than the effective anti-inflammatory dose and so could not be explained by its action as a COX inhibitor. These effects were not blocked by opioid antagonists such as naloxone, and it was eventually discovered that pravadoline represented the first compound from a novel class of cannabinoid agonists, the aminoalkylindoles.Pravadoline was never developed for use as an analgesic, partly due to toxicity concerns (although these were later shown to be a result of the salt form that the drug had been prepared in rather than from the pravadoline itself), however the discovery of cannabinoid activity in this structurally novel family of drugs led to the discovery of several new cannabinoid agonists, including the drug WIN 55,212-2, which is now widely used in scientific research. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Contaminant Sources |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Contaminant Type | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
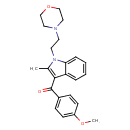
| Chemical Formula | C23H26N2O3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 378.472 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 378.194 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 92623-83-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 3-(4-methoxybenzoyl)-2-methyl-1-[2-(morpholin-4-yl)ethyl]-1H-indole | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | pravadoline | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | COC1=CC=C(C=C1)C(=O)C1=C(C)N(CCN2CCOCC2)C2=CC=CC=C12 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C23H26N2O3/c1-17-22(23(26)18-7-9-19(27-2)10-8-18)20-5-3-4-6-21(20)25(17)12-11-24-13-15-28-16-14-24/h3-10H,11-16H2,1-2H3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | MEUQWHZOUDZXHH-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as benzoylindoles. These are organic compounds containing an indole attached to a benzoyl moiety through the acyl group. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Indoles and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Benzoylindoles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Benzoylindoles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Pravadoline | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 56463 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kegg Compound ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| YMDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECMDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||