| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:27:35 UTC |
|---|
| Update Date | 2016-11-09 01:15:51 UTC |
|---|
| Accession Number | CHEM018691 |
|---|
| Identification |
|---|
| Common Name | Acivicin |
|---|
| Class | Small Molecule |
|---|
| Description | An L-alpha-amino acid that is L-alanine in which the methyl group is replaced by a (5S)-3-chloro-4,5-dihydro-1,2-oxazol-5-yl group. A glutamine analogue antimetabolite, it interferes with glutamate metabolism and several glutamate-dependent synthetic enzymes. It is obtained as a fermentation product of Streptomyces sviceus bacteria. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
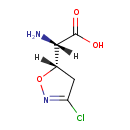
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (alpha-S,5S)-alpha-Amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid | ChEBI | | (AlphaS,5S)-alpha-amino-3-chloro-2-isoxazoline-5-acetic acid | ChEBI | | Acivicine | ChEBI | | Acivicino | ChEBI | | Acivicinum | ChEBI | | Antibiotic at 125 | ChEBI | | AT 125 | ChEBI | | AT-125 | ChEBI | | NSC 163501 | ChEBI | | NSC-163501 | ChEBI | | U 42126 | ChEBI | | U-42,126 | ChEBI | | (a-S,5S)-a-Amino-3-chloro-4,5-dihydro-5-isoxazoleacetate | Generator | | (a-S,5S)-a-Amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid | Generator | | (alpha-S,5S)-alpha-Amino-3-chloro-4,5-dihydro-5-isoxazoleacetate | Generator | | (Α-S,5S)-α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetate | Generator | | (Α-S,5S)-α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid | Generator | | (AlphaS,5S)-a-amino-3-chloro-2-isoxazoline-5-acetate | Generator | | (AlphaS,5S)-a-amino-3-chloro-2-isoxazoline-5-acetic acid | Generator | | (AlphaS,5S)-alpha-amino-3-chloro-2-isoxazoline-5-acetate | Generator | | (AlphaS,5S)-α-amino-3-chloro-2-isoxazoline-5-acetate | Generator | | (AlphaS,5S)-α-amino-3-chloro-2-isoxazoline-5-acetic acid | Generator | | L-(AlphaS,5S)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid | MeSH | | alpha-S,5S-alpha-amino-3-chloro-2-Isoxazoline-5-acetic acid | MeSH | | alpha-amino-3-chloro-4,5-dihydro-5-Isoxazoleacetic acid | MeSH |
|
|---|
| Chemical Formula | C5H7ClN2O3 |
|---|
| Average Molecular Mass | 178.574 g/mol |
|---|
| Monoisotopic Mass | 178.015 g/mol |
|---|
| CAS Registry Number | 42228-92-2 |
|---|
| IUPAC Name | (2S)-2-amino-2-[(5S)-3-chloro-4,5-dihydro-1,2-oxazol-5-yl]acetic acid |
|---|
| Traditional Name | acivicin |
|---|
| SMILES | [H][C@@](N)(C(O)=O)[C@]1([H])CC(Cl)=NO1 |
|---|
| InChI Identifier | InChI=1S/C5H7ClN2O3/c6-3-1-2(11-8-3)4(7)5(9)10/h2,4H,1,7H2,(H,9,10)/t2-,4-/m0/s1 |
|---|
| InChI Key | QAWIHIJWNYOLBE-OKKQSCSOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Isoxazoline
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Amine
- Hydrocarbon derivative
- Organic oxide
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organopnictogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01u0-2900000000-76e795e9bd82e5e43967 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-2900000000-a228ee07a7380aa3da3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fb9-9200000000-54e3b8fb1dc803d75f8f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1900000000-81c845867113a4174dfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-3900000000-e5a8dbb10849b5f0fc08 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9000000000-6cb45d6cb526b41df671 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD0-1036 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Acivicin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 74545 |
|---|
| PubChem Compound ID | 294641 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|