| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:27:07 UTC |
|---|
| Update Date | 2016-11-09 01:15:51 UTC |
|---|
| Accession Number | CHEM018685 |
|---|
| Identification |
|---|
| Common Name | Guaiazulene |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
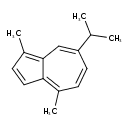
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,4-Dimethyl-7-(1-methylethyl)azulene | ChEBI | | 1,4-Dimethyl-7-isopropylazulene | ChEBI | | 3,8-Dimethyl-5-(2-propyl)azulene | ChEBI | | Guaiazulene | Kegg | | Azunol | Kegg | | Guajazulene | MeSH | | Azulon | MeSH | | 1, 4-Dimethyl-7-isopropylazulene | HMDB | | 1,3,5,7,9-Guaiapentaene | HMDB | | 1,4-Dimethyl-7-(1-methyl)-azulene (azulon) | HMDB | | 1,4-Dimethyl-7-(1-methylethyl)-azulene | HMDB | | 1,4-Dimethyl-7-(1-methylethyl)azulene, 9ci | HMDB | | 1,4-Dimethyl-7-(propan-2-yl)azulene | HMDB | | 1,4-Dimethyl-7-isopropyl-azulene | HMDB | | 7-Isopropyl- 1,4-dimethylazulene | HMDB | | 7-Isopropyl-1,4-dimethyl-azulene | HMDB | | AZ-8 beris | HMDB | | Azulen-beris | HMDB | | Azulene, 7-isopropyl-1,4-dimethyl- (8ci) | HMDB | | Azulol | HMDB | | Cuteazul | HMDB | | Eucazulen | HMDB | | Eucazulene | HMDB | | Guiazulene | HMDB | | Gurjunazulen | HMDB | | Hepatoprotectant | HMDB | | Kessazulen | HMDB | | Kessazulene | HMDB | | Purazulen | HMDB | | S-Guaiazulene | HMDB | | Silazulon | HMDB | | Uroazulen | HMDB | | Vaumigan | HMDB | | Vetivazulen | HMDB | | 7-Isopropyl-1,4-dimethylazulene | ChEBI |
|
|---|
| Chemical Formula | C15H18 |
|---|
| Average Molecular Mass | 198.303 g/mol |
|---|
| Monoisotopic Mass | 198.141 g/mol |
|---|
| CAS Registry Number | 489-84-9 |
|---|
| IUPAC Name | 1,4-dimethyl-7-(propan-2-yl)azulene |
|---|
| Traditional Name | azulon |
|---|
| SMILES | CC(C)C1=CC2=C(C)C=CC2=C(C)C=C1 |
|---|
| InChI Identifier | InChI=1S/C15H18/c1-10(2)13-7-5-11(3)14-8-6-12(4)15(14)9-13/h5-10H,1-4H3 |
|---|
| InChI Key | FWKQNCXZGNBPFD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as guaianes. These are sesquiterpenoids with a structure based on the guaiane skeleton. Guaiane is a bicyclic compound consisting of a decahydroazulene moiety, substituted with two methyl groups and a 1-methylethyl group at the 1-, 4-, and 7-position, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Guaianes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Guaiane sesquiterpenoid
- Azulene
- Aromatic hydrocarbon
- Polycyclic hydrocarbon
- Unsaturated hydrocarbon
- Hydrocarbon
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053r-1900000000-be4c0960d06735436fe8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-3339d5b27a9967e9c5da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-44d06797d9da8aaae9dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05o0-1900000000-6fe86a22e14c459ce6d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-48f7566ceaa93d43c699 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-ee4c5f9ee7e99d6db7ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05o1-1900000000-e4e77ed98ea343b05eb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-5b7ed2802511ed884e20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052b-0900000000-0415b7647d084754c1b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fc0-3900000000-4121eb3d95203bd3b5ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-77af6775e009aeaad051 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-724c6ec495f7c5a30ebc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0900000000-998e7ebc0a1ed3713a46 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB13329 |
|---|
| HMDB ID | HMDB0036648 |
|---|
| FooDB ID | FDB015573 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003138 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 3395 |
|---|
| ChEBI ID | 5550 |
|---|
| PubChem Compound ID | 3515 |
|---|
| Kegg Compound ID | C09675 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|