| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:25:42 UTC |
|---|
| Update Date | 2016-11-09 01:15:51 UTC |
|---|
| Accession Number | CHEM018666 |
|---|
| Identification |
|---|
| Common Name | Cepharanthine |
|---|
| Class | Small Molecule |
|---|
| Description | A bisbenzylisoquinoline alkaloid from tubers of Stephania; stimulates recovery of immunologic function in lymphatic system after administration of antineoplastic agents or x-irradiation. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
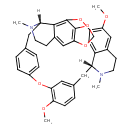
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Cepharanthine | ChEBI | | 6',12'-Dimethoxy-2,2'-dimethyl-6,7-(methylenebis(oxy))oxyacanthan | ChEBI | | Cepharanthin | MeSH |
|
|---|
| Chemical Formula | C37H38N2O6 |
|---|
| Average Molecular Mass | 606.719 g/mol |
|---|
| Monoisotopic Mass | 606.273 g/mol |
|---|
| CAS Registry Number | 481-49-2 |
|---|
| IUPAC Name | (14S,27R)-22,33-dimethoxy-13,28-dimethyl-2,5,7,20-tetraoxa-13,28-diazaoctacyclo[25.6.2.2¹⁶,¹⁹.1³,¹⁰.1²¹,²⁵.0⁴,⁸.0³¹,³⁵.0¹⁴,³⁹]nonatriaconta-1(33),3(39),4(8),9,16,18,21,23,25(36),31,34,37-dodecaene |
|---|
| Traditional Name | cepharanthine |
|---|
| SMILES | [H][C@]12CC3=CC(OC4=CC=C(C[C@]5([H])N(C)CCC6=CC7=C(OCO7)C(OC7=C(OC)C=C(CCN1C)C2=C7)=C56)C=C4)=C(OC)C=C3 |
|---|
| InChI Identifier | InChI=1S/C37H38N2O6/c1-38-13-11-24-18-31(41-4)33-20-27(24)28(38)16-23-7-10-30(40-3)32(17-23)44-26-8-5-22(6-9-26)15-29-35-25(12-14-39(29)2)19-34-36(37(35)45-33)43-21-42-34/h5-10,17-20,28-29H,11-16,21H2,1-4H3/t28-,29+/m1/s1 |
|---|
| InChI Key | YVPXVXANRNDGTA-WDYNHAJCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lignans, neolignans and related compounds. These are plant products of low molecular weight formed primarily from oxidative coupling of two p-propylphenol moieties. They can also be described as micromolecules with two phenylpropanoid units coupled together. They can be attached in various manners, like C5-C5', C8-C8'. Most known natural lignans are oxidized at C9 and C9´ and, based upon the way in which oxygen is incorporated into the skeleton and on the cyclization patterns, a wide range of lignans of very different structural types can be formed. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lignans, neolignans and related compounds |
|---|
| Class | Not Available |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Lignans, neolignans and related compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxyneolignan skeleton
- Diaryl ether
- Tetrahydroisoquinoline
- Benzodioxole
- Anisole
- Alkyl aryl ether
- Aralkylamine
- Benzenoid
- Tertiary aliphatic amine
- Tertiary amine
- Organoheterocyclic compound
- Oxacycle
- Ether
- Acetal
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000049000-6ec61e9173b7989a269b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0000093000-823085996cc1dcfcdf64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-0000090000-1ebadc331082f12f72f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000019000-b2462767da23bd2644fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000096000-4b225a009cdc62267c89 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0569-1000190000-024efae1e4e08a0e3fbf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00001836 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cepharanthine |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 3546 |
|---|
| PubChem Compound ID | 10206 |
|---|
| Kegg Compound ID | C09391 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|