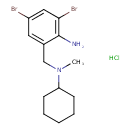
| 2-Amino-N-cyclohexyl-3,5-dibromo-N-methylbenzylamine hydrochloride | ChEBI |
| 3,5-Dibromo-N(alpha)-cyclohexyl-N(alpha)-methyltoluene-alpha,2-diamine monohydrochloride | ChEBI |
| Auxit | ChEBI |
| Bromhexine chloride | ChEBI |
| Bromhexine HCL | ChEBI |
| Bromohexine monohydrochloride | ChEBI |
| Broncokin | ChEBI |
| N-(2-Amino-3,5-dibromobenzyl)-N-methyl-cyclohexylammonium chloride | ChEBI |
| N-(2-Amino-3,5-dibromobenzyl)-N-methylcyclohexanaminium chloride | ChEBI |
| N-Cyclohexyl-N-methyl-(2-amino-3,5-dibromobenzyl)ammonium chloride | ChEBI |
| Ophtolsol | ChEBI |
| Quentan | ChEBI |
| Viscolyt | ChEBI |
| Bisolvon | Kegg |
| 3,5-Dibromo-N(a)-cyclohexyl-N(a)-methyltoluene-a,2-diamine monohydrochloride | Generator |
| 3,5-Dibromo-N(α)-cyclohexyl-N(α)-methyltoluene-α,2-diamine monohydrochloride | Generator |
| 3m Brand OF bromhexine hydrochloride | MeSH |
| Bromhexin berlinchemie | MeSH |
| Bromhexin-ratiopharm | MeSH |
| Brotussol | MeSH |
| Dur elix | MeSH |
| Famel bromhexine | MeSH |
| Hydrochloride, bromhexine | MeSH |
| Monohydrochloride, bromhexine | MeSH |
| SB CH Brand OF bromhexine hydrochloride | MeSH |
| Ratiopharm brand OF bromhexine hydrochloride | MeSH |
| Boehrvet brand OF bromhexine hydrochloride | MeSH |
| Bromhexinratiopharm | MeSH |
| Flegamin | MeSH |
| Merckle brand OF bromhexine hydrochloride | MeSH |
| Novartis brand OF bromhexine hydrochloride | MeSH |
| promeco Brand OF bromhexine hydrochloride | MeSH |
| Boots brand OF bromhexine hydrochloride | MeSH |
| CT-Arzneimittel brand OF bromhexine hydrochloride | MeSH |
| Bromhexine | MeSH |
| Bromhexine hydrochloride | MeSH |
| Flubron | MeSH |
| Krewel brand OF bromhexine hydrochloride | MeSH |
| Mucohexine | MeSH |
| Aparsonin | MeSH |
| BC, Bromhexin | MeSH |
| Berlin-chemie brand OF bromhexine hydrochloride | MeSH |
| Bromhexin BC | MeSH |
| Bromhexin ratiopharm | MeSH |
| Fher brand OF bromhexine hydrochloride | MeSH |
| Hustentabs ratiopharm | MeSH |
| SB-CH Brand OF bromhexine hydrochloride | MeSH |
| CT Arzneimittel brand OF bromhexine hydrochloride | MeSH |
| Apex brand OF bromhexine hydrochloride | MeSH |
| Bromhexin berlin chemie | MeSH |
| Dur-elix | MeSH |
| Tesacof | MeSH |
| Bromhexin von CT | MeSH |
| Von CT, bromhexin | MeSH |
| Berlin chemie brand OF bromhexine hydrochloride | MeSH |
| Bromhexine monohydrochloride | MeSH |
| DurElix | MeSH |
| Hustentabs-ratiopharm | MeSH |
| Hustentabsratiopharm | MeSH |
| Omniapharm brand OF bromhexine hydrochloride | MeSH |
| CT, Bromhexin von | MeSH |
| Boehringer ingelheim brand OF bromhexine hydrochloride | MeSH |
| Bromhexin | MeSH |
| Bromhexin berlin-chemie | MeSH |
| Bromhexine, famel | MeSH |
| Darolan | MeSH |
| Hoechst brand OF bromhexine hydrochloride | MeSH |