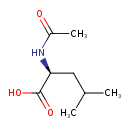
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-000i-6910000000-4832dfe7d6d4267d6e52 | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-002r-6910000000-84a47055ce5d856cbf9e | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-000i-7900000000-a0b6f75cf19418bded48 | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0udi-0930000000-f27350f13ea712c9ca5c | Spectrum |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-000i-6910000000-4832dfe7d6d4267d6e52 | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-002r-6910000000-84a47055ce5d856cbf9e | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-000i-7900000000-a0b6f75cf19418bded48 | Spectrum |
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0udi-0930000000-f27350f13ea712c9ca5c | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9200000000-ad6cdad728e4ab043e38 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-010c-9300000000-ce0116c32d01ba5b766c | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-00di-0900000000-467d952fc32fb0bb8af3 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-001i-0900000000-fdb9099645f68121fb32 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-001i-0900000000-a148fb266810cd2b9b3e | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-001i-5900000000-7b9d62f34c7b6fc2ae4e | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-9000000000-e5aaef8422c96f086069 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - , negative | splash10-001i-0900000000-18d02e7d5844662f8a29 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0adi-0900000000-cedccfd459609718718d | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-000i-9600000000-065fa320d009e309b319 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-000i-9100000000-ba88448ef117528982de | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-000f-9000000000-20376a815d3377cfbd83 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0006-9000000000-dcb83ed9d2b4eb878bc0 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-001i-7900000000-884cd757a0bb6c943f1e | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9300000000-996f7e233ed61eb9f1db | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0006-9000000000-c02e8a0ef249a980c557 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1900000000-7a33d6856c0373f3c0cc | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0006-9000000000-277408d6cc88e96641c7 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-00dr-2900000000-57e7bf1dcfd4157f8a83 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-05mo-9000000000-1f318b754db7ea1fa9ef | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-00gr-2900000000-da6f6595c0d7eaa983e3 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00e9-1900000000-a7e7f933b2155a59555c | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001r-9800000000-f0cf22f10972ee728fe4 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9100000000-598d0e4c77dbf9794ee1 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1900000000-15626e7c74e213c4fb23 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05ai-4900000000-5a450ad57fd8bd9d9f69 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9200000000-f0db45895855a83c4418 | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |