| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:16:30 UTC |
|---|
| Update Date | 2016-11-09 01:15:48 UTC |
|---|
| Accession Number | CHEM018479 |
|---|
| Identification |
|---|
| Common Name | Procaterol |
|---|
| Class | Small Molecule |
|---|
| Description | A long-acting beta-2-adrenergic receptor agonist. It is a potent bronchodilator that may be administered orally or by aerosol inhalation. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
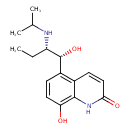
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (R*,s*)-(+-)-8-hydroxy-5-(1-hydroxy-2-((1-methylethyl)amino)butyl)-2(1H)-quinolinone | HMDB | | Pro-air | HMDB | | Procaterol hydrochloride | HMDB | | Procaterol monohydrochloride | HMDB | | Procaterol monohydrochloride, (r*,s*)-(-)-isomer | HMDB | | Hydrochloride, procaterol | HMDB | | Procaterol monohydrochloride, (r*,s*)-(+)-isomer | HMDB | | Procaterol, (r*,r*)-(+-)-isomer | HMDB | | Pro air | HMDB | | ProAir | HMDB | | Monohydrochloride, procaterol | HMDB | | Procaterol monohydrochloride, (r*,r*)-(+)-isomer | HMDB | | Procaterol monohydrochloride, (r*,r*)-(+-)-isomer | HMDB | | Procaterol monohydrochloride, (r*,r*)-(-)-isomer | HMDB | | Procaterol, (r*,s*)-(-)-isomer | HMDB | | Procaterol | MeSH |
|
|---|
| Chemical Formula | C16H22N2O3 |
|---|
| Average Molecular Mass | 290.358 g/mol |
|---|
| Monoisotopic Mass | 290.163 g/mol |
|---|
| CAS Registry Number | 72332-33-3 |
|---|
| IUPAC Name | 8-hydroxy-5-[(1R,2S)-1-hydroxy-2-[(propan-2-yl)amino]butyl]-1,2-dihydroquinolin-2-one |
|---|
| Traditional Name | Pro-Air |
|---|
| SMILES | CC[C@H](NC(C)C)[C@H](O)C1=C2C=CC(=O)NC2=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C16H22N2O3/c1-4-12(17-9(2)3)16(21)11-5-7-13(19)15-10(11)6-8-14(20)18-15/h5-9,12,16-17,19,21H,4H2,1-3H3,(H,18,20)/t12-,16+/m0/s1 |
|---|
| InChI Key | FKNXQNWAXFXVNW-BLLLJJGKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroquinolones. Hydroquinolones are compounds containing a hydrogenated quinoline bearing a ketone group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Quinolones and derivatives |
|---|
| Direct Parent | Hydroquinolones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydroquinolone
- 8-hydroxyquinoline
- Dihydroquinoline
- 1-hydroxy-2-unsubstituted benzenoid
- Pyridinone
- Aralkylamine
- Pyridine
- Benzenoid
- Heteroaromatic compound
- 1,2-aminoalcohol
- Lactam
- Secondary alcohol
- Azacycle
- Secondary aliphatic amine
- Secondary amine
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Organic oxide
- Aromatic alcohol
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0k96-9850000000-0e0d38f482ffc8f878ff | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0l1i-9207500000-609de6c83961abc63757 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0090000000-45681fdc7f6d9b3d4862 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ec-1190000000-e120aada9eb63ff0affe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08fu-9730000000-393abf96681437f84331 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-63f9f3f6d3958d1cd1a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dr-1290000000-1b41d382c48f8cb528e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9530000000-20aca0ea8fe59790ce92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-a6b51079c6e0ef413457 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01qc-2290000000-b724cd9230b3ba331a71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08fu-9430000000-ec4f9cd1f52b26ae03b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-8beee817dd5108cbf1de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-022i-1590000000-a2f858e4c7acfd741391 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01q4-9420000000-ab3184de9bec1620ddbb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01366 |
|---|
| HMDB ID | HMDB0015453 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Procaterol |
|---|
| Chemspider ID | 599984 |
|---|
| ChEBI ID | 1002414 |
|---|
| PubChem Compound ID | 688561 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|