| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:16:23 UTC |
|---|
| Update Date | 2016-11-09 01:15:48 UTC |
|---|
| Accession Number | CHEM018475 |
|---|
| Identification |
|---|
| Common Name | Remoxipride |
|---|
| Class | Small Molecule |
|---|
| Description | An antipsychotic agent that is specific for dopamine D2 receptors. It has been shown to be effective in the treatment of schizophrenia. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
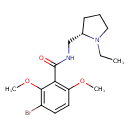
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| FLA-731Roxiam | HMDB | | a-33547REmoxipride | HMDB | | Romoxipride | HMDB | | FLA-731 | HMDB | | Monohydrochloride, remoxipride | HMDB | | Remoxipride hydrochloride | HMDB | | Remoxipride monohydrochloride monohydrate | HMDB | | (S)-3-Bromo-N-((1-ethyl-2-pyrrolidinyl)methyl)-2,6-dimethoxybenzamide | HMDB | | Hydrochloride anhydrous, remoxipride | HMDB | | Remoxipride monohydrochloride | HMDB | | Remoxipride monohydrochloride, (R)-isomer | HMDB | | Remoxipride, (R)-isomer | HMDB | | Remoxipride hydrochloride anhydrous | HMDB | | Anhydrous, remoxipride hydrochloride | HMDB | | Hydrochloride, remoxipride | HMDB | | Monohydrochloride monohydrate, remoxipride | HMDB |
|
|---|
| Chemical Formula | C16H23BrN2O3 |
|---|
| Average Molecular Mass | 371.269 g/mol |
|---|
| Monoisotopic Mass | 370.089 g/mol |
|---|
| CAS Registry Number | 80125-14-0 |
|---|
| IUPAC Name | 3-bromo-N-{[(2S)-1-ethylpyrrolidin-2-yl]methyl}-2,6-dimethoxybenzamide |
|---|
| Traditional Name | remoxipride |
|---|
| SMILES | CCN1CCC[C@H]1CNC(=O)C1=C(OC)C=CC(Br)=C1OC |
|---|
| InChI Identifier | InChI=1S/C16H23BrN2O3/c1-4-19-9-5-6-11(19)10-18-16(20)14-13(21-2)8-7-12(17)15(14)22-3/h7-8,11H,4-6,9-10H2,1-3H3,(H,18,20)/t11-/m0/s1 |
|---|
| InChI Key | GUJRSXAPGDDABA-NSHDSACASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dimethoxybenzenes. These are organic aromatic compounds containing a monocyclic benzene moiety carrying exactly two methoxy groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Methoxybenzenes |
|---|
| Direct Parent | Dimethoxybenzenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - M-dimethoxybenzene
- Dimethoxybenzene
- Halobenzoic acid or derivatives
- 3-halobenzoic acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Phenoxy compound
- Anisole
- Benzoyl
- Phenol ether
- Alkyl aryl ether
- Halobenzene
- Bromobenzene
- Aryl bromide
- Aryl halide
- N-alkylpyrrolidine
- Pyrrolidine
- Amino acid or derivatives
- Carboxamide group
- Secondary carboxylic acid amide
- Tertiary amine
- Tertiary aliphatic amine
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Ether
- Organonitrogen compound
- Organooxygen compound
- Amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organobromide
- Organic oxygen compound
- Organopnictogen compound
- Organohalogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-9021000000-9daaecbf42f2128e1ad4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0519000000-3d2acd15eff0b46d38f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-3912000000-e22b0c0384636903281f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01qc-9110000000-0ddfc4805450139713b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0029000000-cd8c03d7ed0101f27c5c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0294000000-26c857c4f2362cbb3231 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06r6-9671000000-9edffc6cb1f1ba0b83f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0019000000-1785aaf5dd85924ca1a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1329000000-f2461d27117d29c1a137 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01oy-5790000000-c002f172028926a46b44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0029000000-b9eb29b968cffb6427c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-3189000000-e1209bfcec679b882747 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fbc-9072000000-004d419109fc829dd6cb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00409 |
|---|
| HMDB ID | HMDB0014553 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Remoxipride |
|---|
| Chemspider ID | 49195 |
|---|
| ChEBI ID | 127616 |
|---|
| PubChem Compound ID | 54477 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|