| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:15:43 UTC |
|---|
| Update Date | 2016-11-09 01:15:48 UTC |
|---|
| Accession Number | CHEM018450 |
|---|
| Identification |
|---|
| Common Name | Homochlorcyclizine |
|---|
| Class | Small Molecule |
|---|
| Description | Homochlorcyclizine is a diphenylmethylpiperazine H1-antihistamine.↵ |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
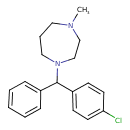
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Curosajin | HMDB | | (±)-homochlorcyclizine | HMDB | | Homochlorocyclizine | HMDB | | Homoclomine | HMDB | | Homodamon | HMDB | | Homorestar | HMDB | | Lysilan | HMDB | | N-(p-Chlorobenzhydryl)-n'-methylhomopiperazine | HMDB | | NSC 25132 | HMDB | | SA 97 | HMDB | | Sankumin | HMDB | | Wicron | HMDB | | Homochlorcyclizine HCL | HMDB | | Homochlorocyclizine hydrochloride | HMDB | | Homochlorocyclizine monohydrochloride | HMDB | | 1-((4-Chlorophenyl)phenylmethyl)hexahydro-4-methyl-1H-1,4-diazepine | HMDB | | Homochlorcyclizine | HMDB, MeSH |
|
|---|
| Chemical Formula | C19H23ClN2 |
|---|
| Average Molecular Mass | 314.860 g/mol |
|---|
| Monoisotopic Mass | 314.155 g/mol |
|---|
| CAS Registry Number | 848-53-3 |
|---|
| IUPAC Name | 1-[(4-chlorophenyl)(phenyl)methyl]-4-methyl-1,4-diazepane |
|---|
| Traditional Name | homochlorcyclizine |
|---|
| SMILES | CN1CCCN(CC1)C(C1=CC=CC=C1)C1=CC=C(Cl)C=C1 |
|---|
| InChI Identifier | InChI=1S/C19H23ClN2/c1-21-12-5-13-22(15-14-21)19(16-6-3-2-4-7-16)17-8-10-18(20)11-9-17/h2-4,6-11,19H,5,12-15H2,1H3 |
|---|
| InChI Key | WEUCDJCFJHYFRL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylmethanes |
|---|
| Direct Parent | Diphenylmethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diphenylmethane
- 1,4-diazepane
- Chlorobenzene
- Diazepane
- Halobenzene
- Aralkylamine
- Aryl chloride
- Aryl halide
- Tertiary aliphatic amine
- Tertiary amine
- Organoheterocyclic compound
- Azacycle
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0029000000-8a61986cf6d60bf81cda | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gb9-3096000000-959bed2bb20cae706198 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-9240000000-e1c510f4600b8a191aa9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-dc2748d860fa60954b1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0159000000-65f5c34b0b35e0ac26b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02fx-5490000000-940d537678147878c1c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0039000000-e5538a12567efbc3dfa8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-0094000000-a3c4471119f072f6e962 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1290000000-05fb262b2cc10b1e2916 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-c54768588510ddbc9bef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ik9-0089000000-970bf509629d7033eebd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ik9-1491000000-b2affbdb4464c5870a0d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0240243 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Homochlorcyclizine |
|---|
| Chemspider ID | 3501 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 3627 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|