| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:15:33 UTC |
|---|
| Update Date | 2016-11-09 01:15:48 UTC |
|---|
| Accession Number | CHEM018444 |
|---|
| Identification |
|---|
| Common Name | Oxibendazole |
|---|
| Class | Small Molecule |
|---|
| Description | Oxibendazole is a polymerase inhibitor in phase III trials for the treatment of helminth intestinal infections. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
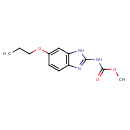
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Anthelcide eq | Kegg | | Methyl 5-N-propoxy-2-benzimidazolecarbamate | MeSH | | N-(6-Propoxy-1H-1,3-benzodiazol-2-yl)methoxycarboximidate | Generator |
|
|---|
| Chemical Formula | C12H15N3O3 |
|---|
| Average Molecular Mass | 249.266 g/mol |
|---|
| Monoisotopic Mass | 249.111 g/mol |
|---|
| CAS Registry Number | 20559-55-1 |
|---|
| IUPAC Name | methyl N-(6-propoxy-1H-1,3-benzodiazol-2-yl)carbamate |
|---|
| Traditional Name | oxibendazole |
|---|
| SMILES | CCCOC1=CC2=C(C=C1)N=C(NC(=O)OC)N2 |
|---|
| InChI Identifier | InChI=1S/C12H15N3O3/c1-3-6-18-8-4-5-9-10(7-8)14-11(13-9)15-12(16)17-2/h4-5,7H,3,6H2,1-2H3,(H2,13,14,15,16) |
|---|
| InChI Key | RAOCRURYZCVHMG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-benzimidazolylcarbamic acid esters. These are aromatic heteropolycyclic compounds that contain a carbamic acid ester group, which is N-linked to the C2-atom of a benzimidazole moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzimidazoles |
|---|
| Sub Class | 2-benzimidazolylcarbamic acid esters |
|---|
| Direct Parent | 2-benzimidazolylcarbamic acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-benzimidazolylcarbamic acid ester
- Alkyl aryl ether
- Benzenoid
- Azole
- Imidazole
- Carbamic acid ester
- Heteroaromatic compound
- Carbonic acid derivative
- Ether
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-2940000000-07d46bca293b8e14042c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0gdi-2790000000-36a8e52bd89d68997bca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f7c-2960000000-62893114d2c82c3730e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-5940000000-e073ecbf859858ea3bfb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-5900000000-a444e0e076bef3fdd0b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-066s-3390000000-d96cf5f3af31cd18e658 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0avj-3890000000-22752ed9ccb016bf9d8b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006t-1900000000-a7952acd2dcb6b70ebf6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04910 |
|---|
| HMDB ID | HMDB0255983 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Oxibendazole |
|---|
| Chemspider ID | 4461 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|