| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:15:16 UTC |
|---|
| Update Date | 2016-11-09 01:15:48 UTC |
|---|
| Accession Number | CHEM018435 |
|---|
| Identification |
|---|
| Common Name | Nicergoline |
|---|
| Class | Small Molecule |
|---|
| Description | An ergot derivative that has been used as a cerebral vasodilator and in peripheral vascular disease. It has been suggested to ameliorate cognitive deficits in cerebrovascular disease. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
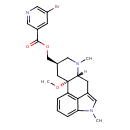
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Sermion | Kegg | | Circo-maren | HMDB | | Dexcel brand OF nicergoline | HMDB | | Nicergolin atid | HMDB | | Nicergolin lindo | HMDB | | Nicergolin-neuraxpharm | HMDB | | Nicergoline hexal brand | HMDB | | Nicerium | HMDB | | Nicotergoline | HMDB | | Pfizer brand OF nicergoline | HMDB | | Teva brand OF nicergoline | HMDB | | CT Arzneimittel brand OF nicergoline | HMDB | | Neuraxpharm brand OF nicergoline | HMDB | | Ratiopharm brand OF nicergoline | HMDB | | Circo maren | HMDB | | Fisifax | HMDB | | Hormosan brand OF nicergoline | HMDB | | Nicergobeta | HMDB | | Nicergolin neuraxpharm | HMDB | | Nicergolin-ratiopharm | HMDB | | Nicergoline pfizer brand | HMDB | | Nicergoline teva brand | HMDB | | Reig jofre brand OF nicergoline | HMDB | | Aventis brand OF nicergoline | HMDB | | F.I. 6714 | HMDB | | Hexal brand OF nicergoline | HMDB | | Lindopharm brand OF nicergoline | HMDB | | Nicergolin-teva | HMDB | | Betapharm brand OF nicergoline | HMDB | | Kenfarma brand OF nicergoline | HMDB | | Krewel brand OF nicergoline | HMDB | | Nicergolin teva | HMDB | | Nicergolin ratiopharm | HMDB | | Nimergoline | HMDB | | CT-Arzneimittel brand OF nicergoline | HMDB | | Ergobel | HMDB | | Nicergolin von CT | HMDB | | Von CT, nicergolin | HMDB |
|
|---|
| Chemical Formula | C24H26BrN3O3 |
|---|
| Average Molecular Mass | 484.386 g/mol |
|---|
| Monoisotopic Mass | 483.116 g/mol |
|---|
| CAS Registry Number | 27848-84-6 |
|---|
| IUPAC Name | [(2S,4R,7R)-2-methoxy-6,11-dimethyl-6,11-diazatetracyclo[7.6.1.0²,⁷.0¹²,¹⁶]hexadeca-1(16),9,12,14-tetraen-4-yl]methyl 5-bromopyridine-3-carboxylate |
|---|
| Traditional Name | nicergoline |
|---|
| SMILES | [H][C@@]12CC3=CN(C)C4=CC=CC(=C34)[C@]1(C[C@@H](COC(=O)C1=CC(Br)=CN=C1)CN2C)OC |
|---|
| InChI Identifier | InChI=1S/C24H26BrN3O3/c1-27-13-17-8-21-24(30-3,19-5-4-6-20(27)22(17)19)9-15(12-28(21)2)14-31-23(29)16-7-18(25)11-26-10-16/h4-7,10-11,13,15,21H,8-9,12,14H2,1-3H3/t15-,21-,24+/m1/s1 |
|---|
| InChI Key | YSEXMKHXIOCEJA-FVFQAYNVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indoloquinolines. These are polycyclic aromatic compounds containing an indole fused to a quinoline. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Indoloquinolines |
|---|
| Direct Parent | Indoloquinolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ergoline skeleton
- Indoloquinoline
- Benzoquinoline
- Pyrroloquinoline
- N-alkylindole
- 3-alkylindole
- Indole
- Indole or derivatives
- Isoindole or derivatives
- Pyridine carboxylic acid
- Alkaloid or derivatives
- Pyridine carboxylic acid or derivatives
- Aralkylamine
- Aryl bromide
- Aryl halide
- N-methylpyrrole
- Piperidine
- Benzenoid
- Pyridine
- Substituted pyrrole
- Pyrrole
- Heteroaromatic compound
- Tertiary amine
- Tertiary aliphatic amine
- Amino acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Azacycle
- Monocarboxylic acid or derivatives
- Dialkyl ether
- Ether
- Amine
- Organopnictogen compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organohalogen compound
- Organic nitrogen compound
- Organobromide
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01oy-0490000000-9946900f54448caef05c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0070900000-31a0fe890dfcef178a8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0090200000-e7257eb7542660c9b4c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pc0-2390000000-90dd1884dd9a2c36f452 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0110900000-1589cacb0deaf8363131 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0532-0920500000-513d6a319962dbf7cbf3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4j-0920000000-330babe951e9ccb2384a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000900000-2649cd14eb52b3a4655b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0000900000-a0efe44d5c8b6301ef53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0391000000-906dc143d70f8ce61d0e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000900000-b7c2f66e57ef37412e1a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-003r-4100900000-fe5f17d8fb6e1a206cbd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-2900100000-a189dad93ea9944c264f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00699 |
|---|
| HMDB ID | HMDB0014837 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Nicergoline |
|---|
| Chemspider ID | 31373 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 34040 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|