| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:15:11 UTC |
|---|
| Update Date | 2016-11-09 01:15:48 UTC |
|---|
| Accession Number | CHEM018433 |
|---|
| Identification |
|---|
| Common Name | Cefepime chloride hydrochloride hydrate |
|---|
| Class | Small Molecule |
|---|
| Description | A hydrochloride that is the monohydrate of the dihydrochloride salt of cefepime. |
|---|
| Contaminant Sources | - ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
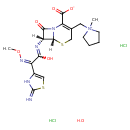
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(((6R,7R)-7-(2-(2-Amino-4-thiazolyl)glyoxylamido)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3- yl)methyl)-1-methylpyrrolidinium chloride, 7(2)-(Z)-(O-methyloxime) monohydrochloride monohydrate | ChEBI | | Cefepime dihydrochloride | ChEBI | | Cefepime dihydrochloride monohydrate | ChEBI | | Cefepime HCL | ChEBI | | Cefepime hydrochloride hydrate | ChEBI | | Cefepime dihydrochloride hydrate | Kegg | | CFPM | Kegg | | Maxipime | Kegg | | 1-(((6R,7R)-7-(2-(2-Amino-4-thiazolyl)glyoxylamido)-2-carboxy-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-3- yl)methyl)-1-methylpyrrolidinium chloride, 7(2)-(Z)-(O-methyloxime) monohydrochloride monohydric acid | Generator | | Cefepime dihydrochloride monohydric acid | Generator | | Cefepime hydrochloride hydric acid | Generator | | Cefepime dihydrochloride hydric acid | Generator | | Axépim | MeSH | | Quadrocef | MeSH | | Cefepim | MeSH | | Cefepime | MeSH | | Bristol-myers brand OF cefepime dihydrochloride | MeSH | | Elan brand OF cefepime dihydrochloride | MeSH | | Bristol-myers squibb brand OF cefepime dihydrochloride | MeSH |
|
|---|
| Chemical Formula | C19H28Cl2N6O6S2 |
|---|
| Average Molecular Mass | 571.490 g/mol |
|---|
| Monoisotopic Mass | 570.089 g/mol |
|---|
| CAS Registry Number | 123171-59-5 |
|---|
| IUPAC Name | 1-{[(6R,7R)-2-carboxylato-7-{[(2Z)-1-hydroxy-2-(2-imino-2,3-dihydro-1,3-thiazol-4-yl)-2-(methoxyimino)ethylidene]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl}-1-methylpyrrolidin-1-ium hydrate dihydrochloride |
|---|
| Traditional Name | cefepim hydrate dihydrochloride |
|---|
| SMILES | O.Cl.Cl.[H][C@]12SCC(C[N+]3(C)CCCC3)=C(N1C(=O)[C@@]2([H])N=C(O)C(=N/OC)\C1=CSC(=N)N1)C([O-])=O |
|---|
| InChI Identifier | InChI=1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1.../s1 |
|---|
| InChI Key | LRAJHPGSGBRUJN-OMIVUECESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha amino acids and derivatives. N-acyl-alpha amino acids and derivatives are compounds containing an alpha amino acid (or a derivative thereof) which bears an acyl group at its terminal nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-alpha amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha amino acid or derivatives
- Cephem
- Meta-thiazine
- N-alkylpyrrolidine
- Azole
- Beta-lactam
- Tetraalkylammonium salt
- Heteroaromatic compound
- Pyrrolidine
- Quaternary ammonium salt
- Tertiary carboxylic acid amide
- Thiazole
- Azetidine
- Carboxamide group
- Carboxylic acid salt
- Isothiourea
- Lactam
- Thioether
- Organic 1,3-dipolar compound
- Organoheterocyclic compound
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Dialkylthioether
- Hemithioaminal
- Carboximidic acid
- Monocarboxylic acid or derivatives
- Carboximidic acid derivative
- Carboxylic acid
- Organic salt
- Organic oxide
- Organic nitrogen compound
- Amine
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic zwitterion
- Hydrochloride
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | Not Available |
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DBSALT000920 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cefepime |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 31368 |
|---|
| PubChem Compound ID | 9571075 |
|---|
| Kegg Compound ID | C12557 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|