| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:11:55 UTC |
|---|
| Update Date | 2016-11-09 01:15:47 UTC |
|---|
| Accession Number | CHEM018379 |
|---|
| Identification |
|---|
| Common Name | Ajmaline |
|---|
| Class | Small Molecule |
|---|
| Description | An alkaloid found in the root of Rauwolfia serpentina, among other plant sources. It is a class Ia antiarrhythmic agent that apparently acts by changing the shape and threshold of cardiac action potentials. Ajmaline produces potent sodium channel blocking effects and a very short half-life which makes it a very useful drug for acute intravenous treatments. The drug has been very popular in some countries for the treatment of atrial fibrillation in patients with the Wolff–Parkinson–White syndrome and in well tolerated monomorphic ventricular tachycardias. It has also been used for many years as a drug to challenge the conduction system of the heart in cases of bundle branch block and syncope. In these cases, abnormal prolongation of the HV interval has been taken as a proof for infrahisian conduction defects tributary for permanent pacemaker implantation. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
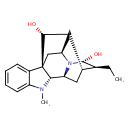
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Ajmaline | ChEBI | | (5AR,6S,8S,10S,11S,11as,12ar,13R)-5-methyl-5a,6,8,9,10,11,11a,12-octahydro-5H-6,10:11,12a-dimethanoindolo[3,2-b]quinolizine-8,13-diol | ChEBI | | Ajimalin | Kegg | | Ajmalin | HMDB |
|
|---|
| Chemical Formula | C20H26N2O2 |
|---|
| Average Molecular Mass | 326.433 g/mol |
|---|
| Monoisotopic Mass | 326.199 g/mol |
|---|
| CAS Registry Number | 4360-12-7 |
|---|
| IUPAC Name | (1R,9R,10S,12R,13S,14R,16S,18R)-13-ethyl-8-methyl-8,15-diazahexacyclo[14.2.1.0¹,⁹.0²,⁷.0¹⁰,¹⁵.0¹²,¹⁷]nonadeca-2,4,6-triene-14,18-diol |
|---|
| Traditional Name | ajmaline |
|---|
| SMILES | CC[C@H]1[C@@H]2C[C@H]3[C@@H]4N(C)C5=CC=CC=C5[C@]44C[C@@H](C2[C@H]4O)N3[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C20H26N2O2/c1-3-10-11-8-14-17-20(12-6-4-5-7-13(12)21(17)2)9-15(16(11)18(20)23)22(14)19(10)24/h4-7,10-11,14-19,23-24H,3,8-9H2,1-2H3/t10-,11-,14-,15-,16?,17-,18+,19+,20+/m0/s1 |
|---|
| InChI Key | CJDRUOGAGYHKKD-HEFSZTOGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ajmaline-sarpagine alkaloids. These are organic compounds containing either of the ajmalan, sarpagan skeleton, or derivative thereof. The Sarpagine (Akuammidine) group, based on the sarpagan nucleus, arises from bond formation between C-16 and C-5 of the corynantheine precursor. Ajmaline alkaloids are based on a 17,19-secoyohimban skeleton (oxayohimban) which is invariably present as an ether. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Ajmaline-sarpagine alkaloids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Ajmaline-sarpagine alkaloids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sarpagine-skeleton
- Beta-carboline
- Pyridoindole
- Quinolizidine
- Indole or derivatives
- Dialkylarylamine
- Quinuclidine
- Tertiary aliphatic/aromatic amine
- Azepane
- Aralkylamine
- Benzenoid
- Piperidine
- Cyclic alcohol
- Secondary alcohol
- Tertiary amine
- Hemiaminal
- Organoheterocyclic compound
- Azacycle
- Alkanolamine
- Alcohol
- Organopnictogen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0bta-2498000000-49bdb2b520b940e4152b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0a59-9006700000-c3e3a75dc9384667f4a8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Ajmaline,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0009000000-852cc00f822b12dde86f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-0029000000-f1807eeec4b4f036d14f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5c-1492000000-fc9be42e8caaa18f3f1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-622c9342ca8dd4c7306c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0029000000-b0778df2272e5a83567c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-055b-0092000000-b4a357ef59beb58eff9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-dae1999fa32e1d569c50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0009000000-26633a00421c4e37223e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002b-0289000000-81cc4c52227dacb33909 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-dac436f1ca5194242739 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0009000000-dac436f1ca5194242739 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0049000000-faf20d5a5170e947fc9b | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01426 |
|---|
| HMDB ID | HMDB0015495 |
|---|
| FooDB ID | FDB030658 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00024294 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ajmaline |
|---|
| Chemspider ID | 10145712 |
|---|
| ChEBI ID | 28462 |
|---|
| PubChem Compound ID | 441080 |
|---|
| Kegg Compound ID | C06542 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|