| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:11:54 UTC |
|---|
| Update Date | 2016-11-09 01:15:47 UTC |
|---|
| Accession Number | CHEM018378 |
|---|
| Identification |
|---|
| Common Name | Disopyramide |
|---|
| Class | Small Molecule |
|---|
| Description | A monocarboxylic acid amide that is butanamide substituted by a diisopropylamino group at position 4, a phenyl group at position 2 and a pyridin-2-yl group at position 2. It is used as a anti-arrhythmia drug. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
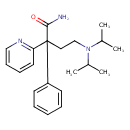
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| alpha-(2-(Diisopropylamino)ethyl)-alpha-phenyl-2-pyridineacetamide | ChEBI | | Disopiramida | ChEBI | | Disopyramidum | ChEBI | | gamma-Diisopropylamino-alpha-phenyl-alpha-(2-pyridyl)butyramide | ChEBI | | Rythmodan p | Kegg | | a-(2-(Diisopropylamino)ethyl)-a-phenyl-2-pyridineacetamide | Generator | | Α-(2-(diisopropylamino)ethyl)-α-phenyl-2-pyridineacetamide | Generator | | g-Diisopropylamino-a-phenyl-a-(2-pyridyl)butyramide | Generator | | Γ-diisopropylamino-α-phenyl-α-(2-pyridyl)butyramide | Generator | | Disopyramide free base | HMDB | | Disopyramide phosphate | HMDB, MeSH | | Diisopyramide | MeSH, HMDB | | Disopyramide phosphate (1:1), (S)-isomer | MeSH, HMDB | | Disopyramide, (+-)-isomer | MeSH, HMDB | | Disopyramide, L-tartrate (1:1), (R)-isomer | MeSH, HMDB | | Disopyramide, L-tartrate (1:1), (S)-isomer | MeSH, HMDB | | Searle brand OF disopyramide phosphate | MeSH, HMDB | | Disopyramide phosphate (1:1), (+-)-isomer | MeSH, HMDB | | Disopyramide phosphate (1:1), (R)-isomer | MeSH, HMDB | | Disopyramide, L-tartrate (1:2), (+-)-isomer | MeSH, HMDB | | Disopyramide, L-tartrate, (S)-isomer | MeSH, HMDB | | Norpace | MeSH, HMDB | | Palpitin | MeSH, HMDB | | Rythmilen | MeSH, HMDB | | Disopyramide phosphate (1:1) | MeSH, HMDB | | Disopyramide, D-tartrate (1:1), (S)-isomer | MeSH, HMDB | | Palpitine | MeSH, HMDB | | Disopyramide monohydrochloride | MeSH, HMDB | | Disopyramide, (R)-isomer | MeSH, HMDB | | Disopyramide, (S)-isomer | MeSH, HMDB | | Rhythmodan | MeSH, HMDB | | Ritmilen | MeSH, HMDB |
|
|---|
| Chemical Formula | C21H29N3O |
|---|
| Average Molecular Mass | 339.475 g/mol |
|---|
| Monoisotopic Mass | 339.231 g/mol |
|---|
| CAS Registry Number | 3737-09-5 |
|---|
| IUPAC Name | 4-[bis(propan-2-yl)amino]-2-phenyl-2-(pyridin-2-yl)butanamide |
|---|
| Traditional Name | disopyramide |
|---|
| SMILES | CC(C)N(CCC(C(N)=O)(C1=CC=CC=C1)C1=NC=CC=C1)C(C)C |
|---|
| InChI Identifier | InChI=1S/C21H29N3O/c1-16(2)24(17(3)4)15-13-21(20(22)25,18-10-6-5-7-11-18)19-12-8-9-14-23-19/h5-12,14,16-17H,13,15H2,1-4H3,(H2,22,25) |
|---|
| InChI Key | UVTNFZQICZKOEM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pheniramines. Pheniramines are compounds containing a pheniramine moiety, which is structurally characterized by the presence of a 2-benzylpyridine linked to an dimethyl(propyl)amine to form a dimethyl[3-phenyl-3-(pyridin-2-yl)propyl]amine skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pheniramines |
|---|
| Direct Parent | Pheniramines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pheniramine
- Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- Heteroaromatic compound
- Tertiary amine
- Tertiary aliphatic amine
- Carboximidic acid derivative
- Carboximidic acid
- Azacycle
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-044m-6591000000-ade312d81f3d174aaf2d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0005-2920000000-e596d22799910844ad71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0019000000-d1d8426d9efe63a8f3fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dj-0295000000-27ad50b3e8f64e45b5f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059l-7590000000-29c623252c76f0cfe4e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000j-0179000000-d43460de1f53211201c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-1491000000-c6005857bfdac87779c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uxr-7920000000-c8e9f71b29cfc8635a28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000f-0049000000-d7931ec90cdb1287fd3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000l-0292000000-4ce9f6848ae81d59c7e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-0900000000-ce002f8854f066661807 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0029000000-6569595ba95c091f548b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000g-8695000000-4b18e01643972a8c9216 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001m-9720000000-fc09dce8697633f796e9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00280 |
|---|
| HMDB ID | HMDB0259629 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Disopyramide |
|---|
| Chemspider ID | 3002 |
|---|
| ChEBI ID | 4657 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C06965 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|