| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:11:03 UTC |
|---|
| Update Date | 2016-11-09 01:15:47 UTC |
|---|
| Accession Number | CHEM018359 |
|---|
| Identification |
|---|
| Common Name | Florfenicol |
|---|
| Class | Small Molecule |
|---|
| Description | A carboxamide that is the N-dichloroacetyl derivative of (1R,2S)-2-amino-3-fluoro-1-propan-1-ol. A synthetic veterinary antibiotic that is used for treatment of bovine respiratory disease and foot rot; also used in aquaculture. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- Suspected Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
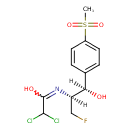
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Florfenicol | ChEBI | | D-Threo-2,2-dichloro-N-(alpha-(fluoromethyl)-beta-hydroxy-p-(methylsulfonyl)phenethyl)acetamide | ChEBI | | Nuflor | ChEBI | | Nuflor gold | ChEBI | | SCH 25298 | ChEBI | | SCH-25298 | ChEBI | | D-Threo-2,2-dichloro-N-(a-(fluoromethyl)-b-hydroxy-p-(methylsulfonyl)phenethyl)acetamide | Generator | | D-Threo-2,2-dichloro-N-(a-(fluoromethyl)-b-hydroxy-p-(methylsulphonyl)phenethyl)acetamide | Generator | | D-Threo-2,2-dichloro-N-(alpha-(fluoromethyl)-beta-hydroxy-p-(methylsulphonyl)phenethyl)acetamide | Generator | | D-Threo-2,2-dichloro-N-(α-(fluoromethyl)-β-hydroxy-p-(methylsulfonyl)phenethyl)acetamide | Generator | | D-Threo-2,2-dichloro-N-(α-(fluoromethyl)-β-hydroxy-p-(methylsulphonyl)phenethyl)acetamide | Generator | | Chloramphen | MeSH | | Thiamphenicol, 3-fluoro | MeSH | | Florphenicol | MeSH | | 3-Fluorothiamphenicol | MeSH | | 2,2-dichloro-N-[(1R,2S)-3-fluoro-1-Hydroxy-1-(4-methylsulphonylphenyl)propan-2-yl]acetamide | Generator | | Florfenicol | MeSH |
|
|---|
| Chemical Formula | C12H14Cl2FNO4S |
|---|
| Average Molecular Mass | 358.210 g/mol |
|---|
| Monoisotopic Mass | 357.000 g/mol |
|---|
| CAS Registry Number | 73231-34-2 |
|---|
| IUPAC Name | 2,2-dichloro-N-[(1R,2S)-3-fluoro-1-hydroxy-1-(4-methanesulfonylphenyl)propan-2-yl]ethanimidic acid |
|---|
| Traditional Name | florfenicol |
|---|
| SMILES | [H][C@@](O)(C1=CC=C(C=C1)S(C)(=O)=O)[C@@]([H])(CF)N=C(O)C(Cl)Cl |
|---|
| InChI Identifier | InChI=1S/C12H14Cl2FNO4S/c1-21(19,20)8-4-2-7(3-5-8)10(17)9(6-15)16-12(18)11(13)14/h2-5,9-11,17H,6H2,1H3,(H,16,18)/t9-,10-/m1/s1 |
|---|
| InChI Key | AYIRNRDRBQJXIF-NXEZZACHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzenesulfonyl compounds. These are aromatic compounds containing a benzenesulfonyl group, which consists of a monocyclic benzene moiety that carries a sulfonyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzenesulfonyl compounds |
|---|
| Direct Parent | Benzenesulfonyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzenesulfonyl group
- Sulfone
- Sulfonyl
- Secondary alcohol
- Carboximidic acid
- Carboximidic acid derivative
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organic oxide
- Aromatic alcohol
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organofluoride
- Organochloride
- Organohalogen compound
- Alkyl chloride
- Alcohol
- Alkyl fluoride
- Alkyl halide
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-053u-2940000000-92c2497527c29726f568 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-053u-2940000000-92c2497527c29726f568 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0329000000-f3c9a6ba314784cec64e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05ds-1975000000-916dcf7c529e8b283f4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06ri-2900000000-4ff799d7ac8e8c247141 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-2349000000-c044673f6ca6bb380940 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-7955000000-bb6e9da1b8d1677f58db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9700000000-474def6bf3be9b152ee1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB11413 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Florfenicol |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 87185 |
|---|
| PubChem Compound ID | 114811 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|