| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-22 05:09:57 UTC |
|---|
| Update Date | 2016-11-09 01:15:47 UTC |
|---|
| Accession Number | CHEM018337 |
|---|
| Identification |
|---|
| Common Name | Apramycin |
|---|
| Class | Small Molecule |
|---|
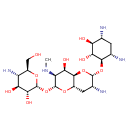
| Description | Apramycin is an aminoglycoside antibiotic and has a bactericidal action against many gram-negative bacteria. Apramycin is a structurally unique antibiotic that contains a bicyclic sugar moiety and a monosubstituted deoxystreptamine. It is not approved for use in humans. |
|---|
| Contaminant Sources | - STOFF IDENT Compounds
- ToxCast & Tox21 Chemicals
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-O-((8R)-2-Amino-8-O-(4-amino-4-deoxy-alpha-D-glucopyranosyl)-2,3,7-trideoxy-7-(methylamino)-D-glycero-alpha-D-allo-octodialdo-1,5:8,4-dipyranos-1-yl)-2-deoxy-D-streptamine | ChEBI | | 4-O-(3alpha-Amino-6alpha-((4-amino-4-deoxy-alpha-D-glucopyranosyl)oxy)-2,3,4,5abeta,6,7,8,8aalpha-octahydro-8beta-hydroxy-7beta-(methylamino)pyrano(3,2-b)pyran-2alpha-yl)-2-deoxy-D-streptamine | ChEBI | | Apramicina | ChEBI | | Apramycine | ChEBI | | Apramycinum | ChEBI | | Nebramycin factor 2 | ChEBI | | Nebramycin II | ChEBI | | 4-O-((8R)-2-Amino-8-O-(4-amino-4-deoxy-a-D-glucopyranosyl)-2,3,7-trideoxy-7-(methylamino)-D-glycero-a-D-allo-octodialdo-1,5:8,4-dipyranos-1-yl)-2-deoxy-D-streptamine | Generator | | 4-O-((8R)-2-Amino-8-O-(4-amino-4-deoxy-α-D-glucopyranosyl)-2,3,7-trideoxy-7-(methylamino)-D-glycero-α-D-allo-octodialdo-1,5:8,4-dipyranos-1-yl)-2-deoxy-D-streptamine | Generator | | 4-O-(3a-Amino-6a-((4-amino-4-deoxy-a-D-glucopyranosyl)oxy)-2,3,4,5abeta,6,7,8,8aalpha-octahydro-8b-hydroxy-7b-(methylamino)pyrano(3,2-b)pyran-2a-yl)-2-deoxy-D-streptamine | Generator | | 4-O-(3Α-amino-6α-((4-amino-4-deoxy-α-D-glucopyranosyl)oxy)-2,3,4,5abeta,6,7,8,8aalpha-octahydro-8β-hydroxy-7β-(methylamino)pyrano(3,2-b)pyran-2α-yl)-2-deoxy-D-streptamine | Generator | | Apramycin sulfate | MeSH |
|
|---|
| Chemical Formula | C21H41N5O11 |
|---|
| Average Molecular Mass | 539.577 g/mol |
|---|
| Monoisotopic Mass | 539.280 g/mol |
|---|
| CAS Registry Number | 37321-09-8 |
|---|
| IUPAC Name | (2R,3R,4S,5S,6S)-2-{[(2R,3S,4R,4aR,6S,7R,8aS)-7-amino-6-{[(1R,2R,3S,4R,6S)-4,6-diamino-2,3-dihydroxycyclohexyl]oxy}-4-hydroxy-3-(methylamino)-octahydropyrano[3,2-b]pyran-2-yl]oxy}-5-amino-6-(hydroxymethyl)oxane-3,4-diol |
|---|
| Traditional Name | apramycin |
|---|
| SMILES | CN[C@H]1[C@@H](O)[C@H]2O[C@H](O[C@@H]3[C@@H](N)C[C@@H](N)[C@H](O)[C@H]3O)[C@H](N)C[C@@H]2O[C@@H]1O[C@H]1O[C@H](CO)[C@@H](N)[C@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C21H41N5O11/c1-26-11-14(30)18-8(33-20(11)37-21-16(32)13(29)10(25)9(4-27)34-21)3-7(24)19(36-18)35-17-6(23)2-5(22)12(28)15(17)31/h5-21,26-32H,2-4,22-25H2,1H3/t5-,6+,7-,8+,9-,10-,11+,12+,13+,14-,15-,16-,17-,18+,19+,20-,21-/m1/s1 |
|---|
| InChI Key | XZNUGFQTQHRASN-XQENGBIVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aminocyclitol glycosides. These are organic compounds containing an amicocyclitol moiety glycosidically linked to a carbohydrate moiety. There are two major classes of aminoglycosides containing a 2-streptamine core. They are called 4,5- and 4,6-disubstituted 2-deoxystreptamines. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Aminocyclitol glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Amino cyclitol glycoside
- Hexose monosaccharide
- Aminocyclitol or derivatives
- Cyclohexylamine
- Cyclohexanol
- Oxane
- Cyclitol or derivatives
- Monosaccharide
- 1,3-aminoalcohol
- Cyclic alcohol
- Secondary alcohol
- 1,2-aminoalcohol
- Oxacycle
- Organoheterocyclic compound
- Secondary amine
- Acetal
- Secondary aliphatic amine
- Organopnictogen compound
- Primary aliphatic amine
- Organonitrogen compound
- Primary alcohol
- Organic nitrogen compound
- Amine
- Alcohol
- Primary amine
- Hydrocarbon derivative
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03mi-0609060000-be32be8bb819fe00bb8f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0903010000-245a139b0e513ad0f210 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03ka-6925000000-5e9c617ea15eb45bbc05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0729-2973240000-f9d27a3b85b6fc213321 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fu-1913010000-0b9c2b857514700bb081 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-114i-7920000000-fa96e884539e16c3ec58 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04626 |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Apramycin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 2790 |
|---|
| PubChem Compound ID | 3081545 |
|---|
| Kegg Compound ID | C01555 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|